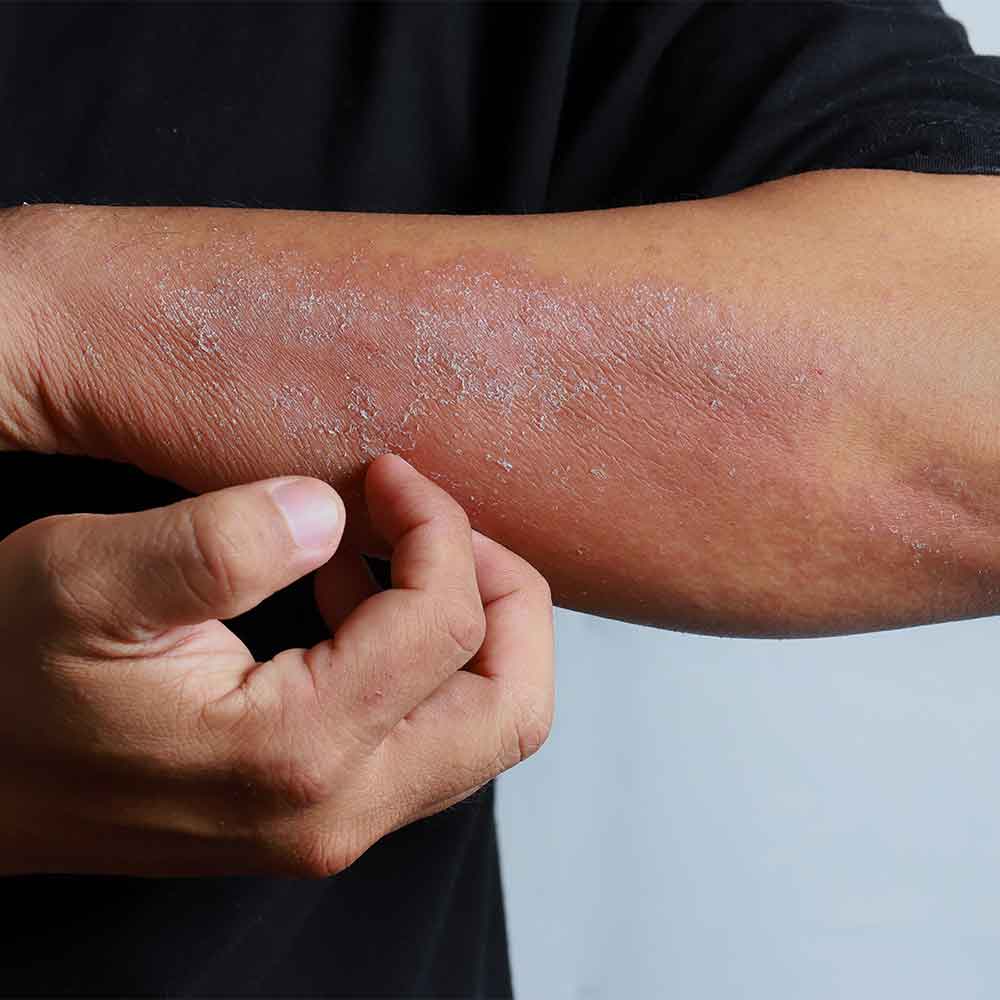
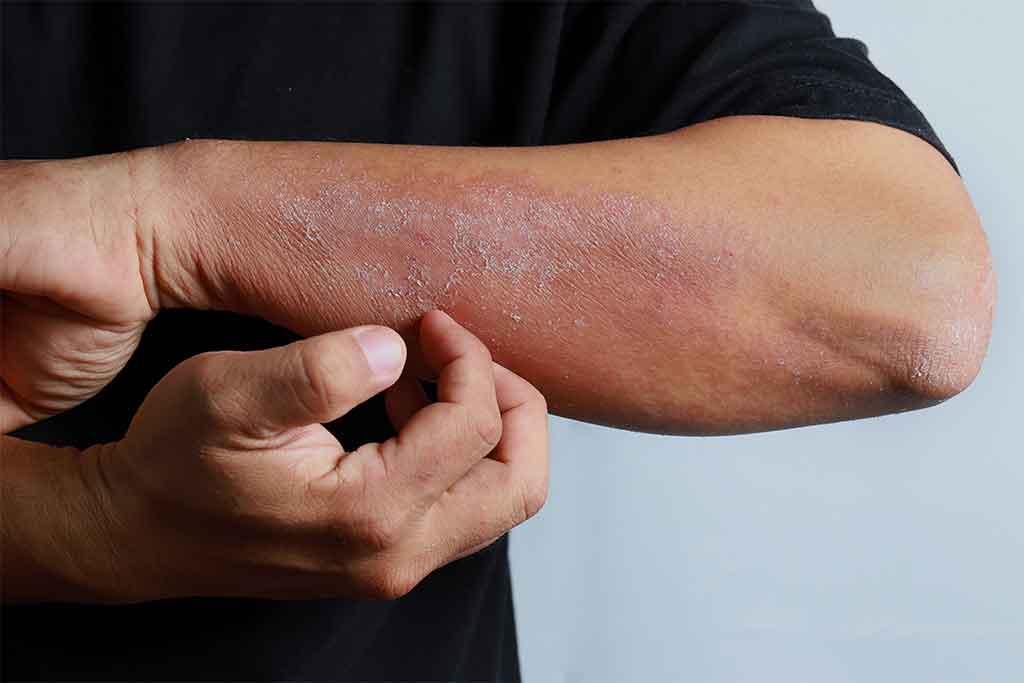
Dupilumab was added to the Pharmaceutical Benefits Scheme (PBS) on 1 March 2021. Dupilumab is indicated for the treatment of moderate to severe atopic dermatitis and asthma in people aged 12 years of age and older. However, it is currently only PBS-subsidised for the treatment of chronic severe atopic dermatitis.
Dupilumab is a human monoclonal antibody that acts as an antagonist at the interleukin-4 (IL-4) receptor alpha subunit shared by IL-4 and IL-13 receptors. This results in inhibition of signalling via IL-4 and IL-13, key cytokines involved in atopic disease.
The safety and efficacy of dupilumab in atopic dermatitis were evaluated in three pivotal placebo-controlled clinical trials. The proportion of subjects with an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) and an improvement of at least two points was significantly higher in the dupilumab groups in all three studies. This outcome occurred in 38% of patients in the SOLO 1 trial and 36% of patients in the SOLO 2 trial who received dupilumab every second week, compared to 10% and 8% in the placebo groups, respectively.
Dupilumab is presented as a single-use pre-filled syringe for subcutaneous injection. It is intended for long-term use and can be combined with topical therapy, if required. The most commonly reported adverse reactions include injection site reactions, herpes simplex virus infection, conjunctivitis, and blepharitis.


From 1 March 2021, Atectura® is available on the Pharmaceutical Benefits Scheme (PBS) for the maintenance treatment of asthma in people 12 years of age and older. Atectura® contains the long-acting beta2– agonist (LABA), indacaterol, plus the corticosteroid, mometasone. It is presented as a capsule, the contents of which must be inhaled using the Breezhaler® device supplied in the carton.
The Australian Asthma Handbook advises that, for adult patients already taking an inhaled corticosteroid, the addition of a LABA reduces the risk of exacerbations compared with increasing the corticosteroid dose. The results of the PALLADIUM study support this advice. In this study, medium and high doses of mometasone plus indacaterol demonstrated superior improvements in forced expiratory volume in one second (FEV1) compared to higher doses of mometasone. In addition, high-dose mometasone plus indacaterol was shown to be non-inferior to high-dose fluticasone propionate plus salmeterol in improving FEV1.
Atectura® is available in three mometasone strengths, with each presentation containing 125mcg of indacaterol. Dysphonia and candidiasis were among the most frequent adverse events reported in clinical trials; patients should be advised to rinse their mouth with water after each dose.


The product information for metronidazole intravenous infusion (Pfizer) has recently been updated to include new contraindications. The contraindications now include disulfiram therapy in the previous two weeks, and consumption of alcohol or products containing propylene glycol.
Interactions with disulfiram and alcohol have long been associated with metronidazole, although the incidence is uncertain and the severity varied. Psychotic reactions and confusion have been reported when disulfiram is administered with metronidazole. Disulfiram-like reactions have been described when metronidazole is combined with alcohol or propylene glycol. These reactions may include tachycardia, flushing, or vomiting.
The updated product information recommends that alcohol and products containing propylene glycol be discontinued during metronidazole therapy and for at least three days after the last metronidazole dose. Avoiding these agents requires some care as alcohol and propylene glycol are excipients in many medications.
Medications containing ethanol and propylene glycol include:
- Diazepam for injection;
- Phenobarbital (phenobarbitone) injection;
- Phenytoin injection;
- Glyceryl trinitrate injection;
- Digoxin (Lanoxin® Paediatric Elixir, Lanoxin® Paediatric Injection, Lanoxin® Adult Injection); and
- Lopinavir + ritonavir (Kaletra®) oral solution.
Medicines that may contain ethanol include:
- Ranitidine oral liquid;
- Furosemide oral solution;
- Trimethoprim + sulfamethoxazole oral liquid; and
- Iron liquids (e.g. Maltofer® Syrup)
The approved product information should be reviewed to determine if a medicine contains ethanol or propylene glycol.


Ripretinib is a switch-control tyrosine kinase inhibitor recently approved by the Therapeutic Goods Administration (TGA). It is indicated for the treatment of advanced gastrointestinal stromal tumours (GIST) in adults who have previously been treated with imatinib and at least two other kinase inhibitors. This approval addresses a previously unmet clinical need as there are no other approved therapies for patients with advanced or unresectable GIST who experience disease progression following treatment with imatinib, sunitinib, and regorafenib.
Ripretinib inhibits KIT proto-oncogene and platelet-derived growth factor receptor α (PDGFRA) kinase activity. Most GISTs have activating mutations in these kinases that may cause dysregulated cell growth. Ripretinib can transform the kinase into an inactive conformation which prevents downstream signalling and cell proliferation. It is also active against a broad spectrum of KIT and PDGFRA mutations.
The efficacy of ripretinib is supported by the INVICTUS study. This randomised, double-blind trial investigated ripretinib as a fourth-line agent in patients with advanced GIST. The median progression-free survival was 6.3 months in the ripretinib group, compared to 1.0 months in the placebo group. The most common adverse effects associated with ripretinib were alopecia, myalgia, nausea, fatigue, palmar-plantar erythrodysaesthesia syndrome, and diarrhoea.
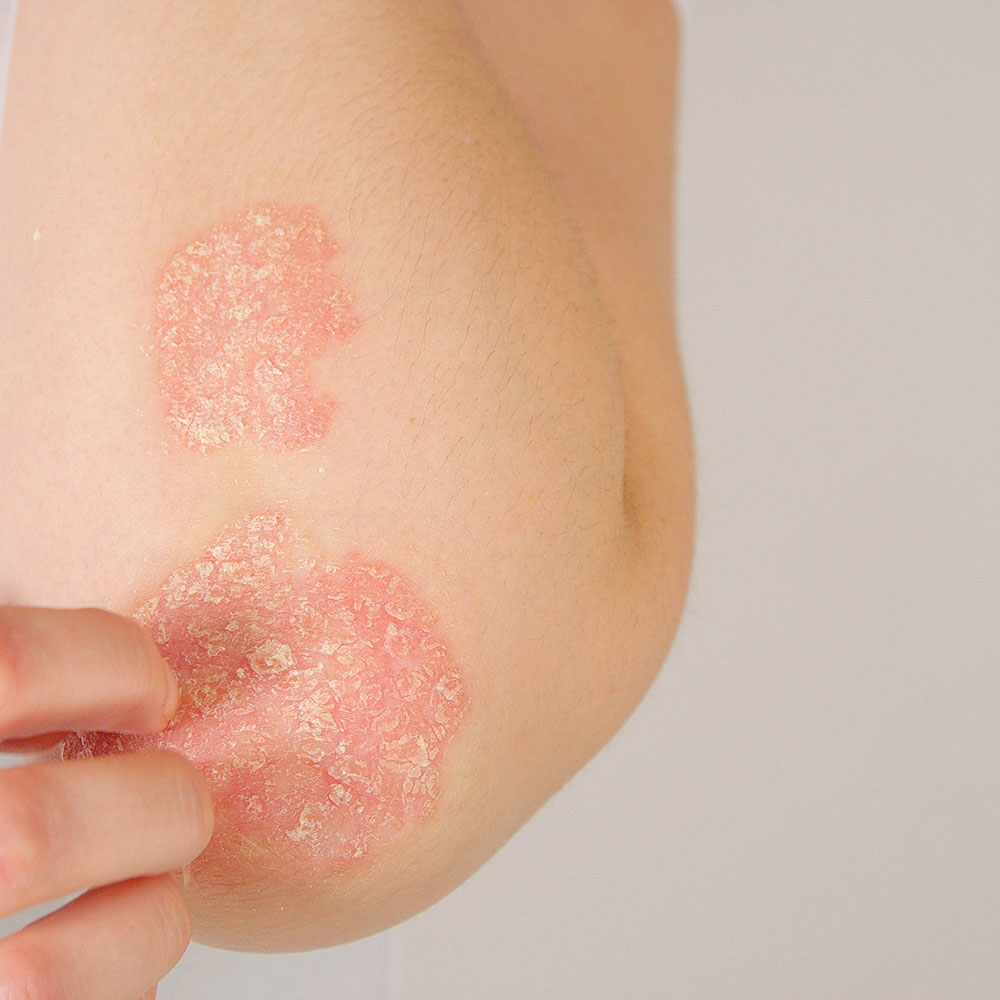
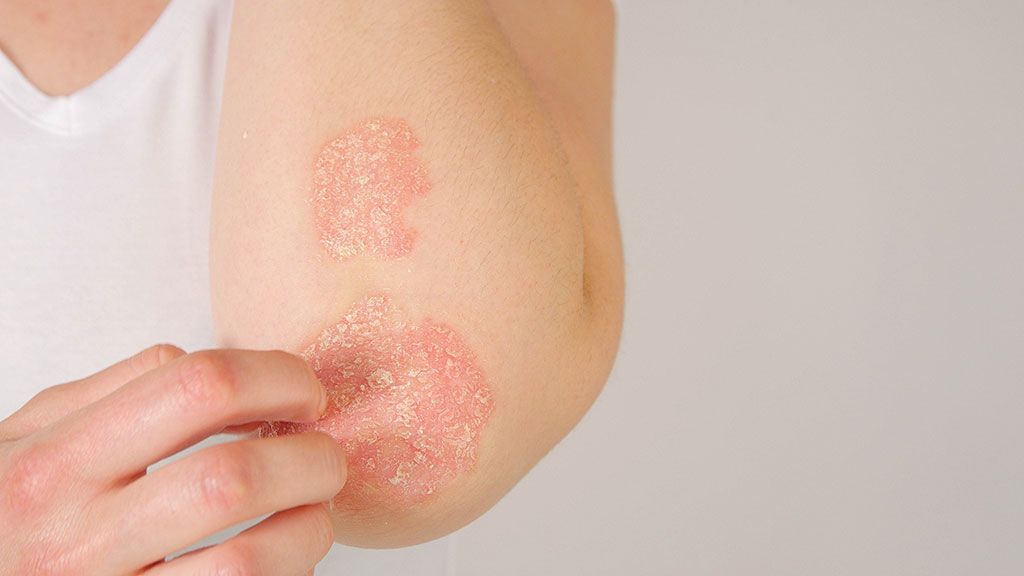
From 1 January 2021, apremilast is available on the Pharmaceutical Benefits Scheme (PBS). Apremilast is PBS listed for the treatment of severe chronic plaque psoriasis that has a significant effect on quality of life. Patients must be unable to take methotrexate or have achieved an inadequate response following at least six weeks of methotrexate therapy.
Apremilast is a phosphodiesterase 4 (PDE4) inhibitor. Inhibition of PDE4 increases cyclic adenosine monophosphate (cAMP), an important regulator of innate and adaptive immune functions. The result is reduced production of inflammatory cytokines such as interleukin (IL)-17, IL-23, and tumour necrosis factor-alpha (TNF-α), and an increase in anti-inflammatory mediators such as IL-10.
The safety and efficacy of apremilast were studied in the ESTEEM-2 trial. Significantly more patients in the apremilast group achieved ≥75% improvement in their Psoriasis Area and Severity Index (PASI) score compared to placebo (28.8% versus 5.8%). The mean change in PASI score was -50.9% for apremilast compared to -15.8% for placebo. The most commonly reported adverse events were diarrhoea and nausea. The apremilast dosing schedule includes an initial titration period which is intended to minimise gastrointestinal adverse effects.


Dapagliflozin has recently been approved for the treatment of symptomatic heart failure. Dapagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor originally only indicated for the management of type 2 diabetes mellitus.
Initial safety trials of dapagliflozin suggested a reduced cardiovascular risk, which was further investigated in the DAPA-HF Trial. In this trial, patients with heart failure and an ejection fraction of ≤40% were randomly assigned to receive dapagliflozin 10mg daily or placebo, in addition to standard therapy. The primary outcome, a composite of worsening heart failure or cardiovascular death, occurred in 16.3% of the dapagliflozin group compared to 21.2% of the placebo group. There was no significant difference between the two groups in the incidence of adverse effects related to volume depletion, renal dysfunction, or hypoglycaemia. Interestingly, the cardiovascular benefits of dapagliflozin were observed regardless of diabetes status.
For the treatment of heart failure, dapagliflozin should be administered in conjunction with individualised standard of care therapy. Dapagliflozin is currently only subsidised on the Pharmaceutical Benefits Scheme (PBS) for the treatment of type 2 diabetes mellitus.


A new fixed-dose combination inhaler has been added to the Pharmaceutical Benefits Scheme (PBS). Fostair® contains the corticosteroid, beclometasone and the long-acting beta2 agonist, formoterol. It is PBS-subsidised for adults who have previously had frequent episodes of asthma while receiving treatment with oral corticosteroids or optimal doses of inhaled corticosteroids. Fostair® is also approved for the symptomatic treatment of severe chronic obstructive pulmonary disease (COPD), although this is not currently a PBS-subsidised indication.
Fostair® contains beclometasone and formoterol with an extra-fine particle size distribution. A study conducted in patients with moderate to severe asthma demonstrated that Fostair® is more effective at improving lung function than a double equipotent dosage of non-extra-fine beclometasone. In more severe asthma, studies demonstrated that Fostair® is as effective in improving lung function as the two individual components administered separately and superior to non-extra-fine beclometasone alone.
There are two approved treatment approaches for asthma: maintenance therapy and maintenance and reliever therapy. Patients taking Fostair® as maintenance therapy should have a separate rapid-acting bronchodilator to use when required for acute symptoms. For maintenance and reliever therapy, Fostair® is used both regularly for maintenance treatment and as needed in response to asthma symptoms. However, Fostair® is not PBS-subsidised for maintenance and reliever therapy.


From 1 December 2020, rivaroxaban 2.5mg tablets are available on the Pharmaceutical Benefits Scheme (PBS) for chronic stable atherosclerotic disease. The PBS criteria requires that rivaroxaban 2.5mg be used in combination with aspirin in high-risk patients with coronary artery disease or peripheral artery disease.
Evidence to support the use of rivaroxaban 2.5mg in this patient group comes from the COMPASS trial. Participants with stable atherosclerotic vascular disease were randomly assigned to receive rivaroxaban 2.5mg twice daily plus aspirin 100mg daily, rivaroxaban 5mg daily, or aspirin 100mg daily. The primary efficacy outcome was the composite of cardiovascular death, stroke, or myocardial infarction. This occurred in 4.1% of the rivaroxaban plus aspirin group, 4.9% of the rivaroxaban alone group, and 5.4% of the aspirin alone group.
There was no significant difference in the rate of intracranial or fatal bleeding events between groups. However, major bleeding events were more common in the rivaroxaban plus aspirin group (3.1% compared to 1.9% in the aspirin alone group). The study was stopped after a mean follow-up of 23 months due to the superiority of rivaroxaban plus aspirin.


The Therapeutic Goods Administration (TGA) has investigated metformin-containing medicines for potential contamination with N-nitrosodimethylamine (NDMA). NDMA is classified as a probable human carcinogen and can be found in low levels in a variety of foods, some drinking water, and air pollution. It has also recently been detected in some ranitidine and angiotensin II antagonist medicines.
Testing of a selection of immediate-release and modified-release metformin products found that around 30% of batches contained NDMA at levels that modestly exceeded the acceptable limit. While one batch was found to have higher levels, limited stock of this product was supplied to the Australian market.
A risk analysis was conducted that included consideration of commonly prescribed doses. The TGA concludes that the risk is very low for patients taking metformin from batches that modestly exceed the NDMA limit. The limits set for NDMA are conservative and calculated so that an individual’s excess cancer risk would not exceed 1:100,000 if they received the maximum daily dose of that medicine for 70 years.
Metformin is an important treatment for type 2 diabetes. The TGA advises that the risks of untreated diabetes greatly outweigh the risks posed by the low levels of NDMA detected in these products. Patients are strongly recommended to continue taking their medication as prescribed. The TGA is currently working with manufacturers and sponsors to improve processes and ensure that metformin products supplied in Australia meet the TGA’s high standards for quality.


From 1 November 2020, siponimod became available on the Pharmaceutical Benefits Scheme (PBS) for the treatment of relapsing-remitting multiple sclerosis. Siponimod is a sphingosine-1-phosphate (S1P) receptor modulator. It acts as a functional antagonist at S1P1 receptors to inhibit the release of lymphocytes from the lymph nodes. This may reduce lymphocyte migration to the central nervous system and minimise central inflammation.
A large double-blind clinical trial demonstrates that siponimod reduces disability progression and has a similar safety profile to fingolimod. The primary endpoint of time to onset of confirmed disability progression (CDP) at three months was significantly delayed, with a relative risk reduction of 21% (CDP events occurred in 26% of siponimod patients, compared to 32% of the placebo group). Adverse events most commonly reported during this trial include elevated liver enzymes, bradycardia at initiation, macular oedema, hypertension, varicella-zoster virus reactivation, and convulsions.
Siponimod is extensively metabolised by the cytochrome P450 system, primarily by CYP2C9. Patients should be tested for their CYP2C9 metabolising enzyme status before commencing treatment. Dose reductions are required for patients with CYP2C9*1*3 or CYP2C9*2*3 genotypes, while use is contraindicated in patients with a CYP2C9*3*3 genotype as substantially higher plasma levels will occur.




















