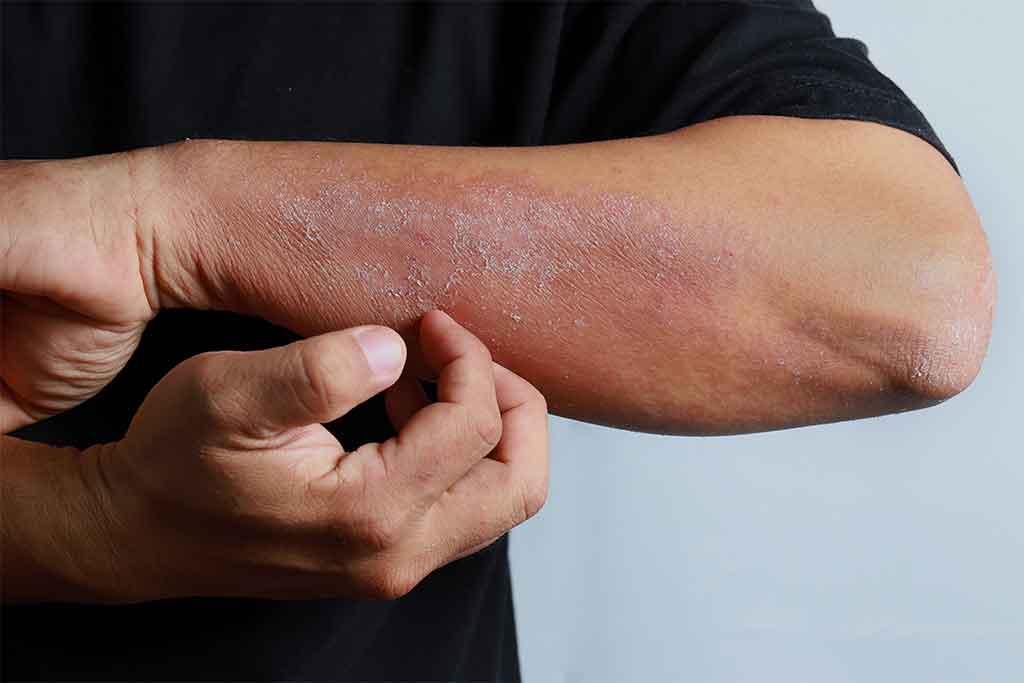
Dupilumab was added to the Pharmaceutical Benefits Scheme (PBS) on 1 March 2021. Dupilumab is indicated for the treatment of moderate to severe atopic dermatitis and asthma in people aged 12 years of age and older. However, it is currently only PBS-subsidised for the treatment of chronic severe atopic dermatitis.
Dupilumab is a human monoclonal antibody that acts as an antagonist at the interleukin-4 (IL-4) receptor alpha subunit shared by IL-4 and IL-13 receptors. This results in inhibition of signalling via IL-4 and IL-13, key cytokines involved in atopic disease.
The safety and efficacy of dupilumab in atopic dermatitis were evaluated in three pivotal placebo-controlled clinical trials. The proportion of subjects with an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) and an improvement of at least two points was significantly higher in the dupilumab groups in all three studies. This outcome occurred in 38% of patients in the SOLO 1 trial and 36% of patients in the SOLO 2 trial who received dupilumab every second week, compared to 10% and 8% in the placebo groups, respectively.
Dupilumab is presented as a single-use pre-filled syringe for subcutaneous injection. It is intended for long-term use and can be combined with topical therapy, if required. The most commonly reported adverse reactions include injection site reactions, herpes simplex virus infection, conjunctivitis, and blepharitis.
References:
- Department of Health. Public Summary Document: Dupilumab – March 2020 PBAC Meeting. Canberra: Pharmaceutical Benefits Scheme; 2020.
- Dupixent® (Dupilumab) Australian approved product information. Macquarie Park: Sanofi-Aventis Australia. Approved October 2020.
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016; 375: 2335-48.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
