

Pembrolizumab is now available on the Pharmaceutical Benefits Scheme (PBS) for the first-line treatment of metastatic non-small cell lung cancer (NSCLC) irrespective of programmed death ligand-1 (PD-L1) status.
In the KEYNOTE-189 trial, pembrolizumab was used in conjunction with pemetrexed and platinum chemotherapy in metastatic NSCLC irrespective of PD-L1 status, opening up a new standard of care for these patients. The median duration follow-up was 18.7 months, and pembrolizumab was associated with a significantly longer overall survival (22.0 versus 10.7 months) compared to pemetrexed and platinum-based chemotherapy alone.
The incidence of treatment-related adverse effects was similar between the treatment arms. However, immune-mediated adverse effects including hypothyroidism and hyperthyroidism, pneumonitis, skin toxicity, colitis and infusion reactions, were markedly higher with the pembrolizumab combination at 22.7% and chemotherapy at 11.9%.
An updated edition of the Therapeutic Guidelines: Oral and Dental has recently been released. This resource covers a broad range of conditions that are relevant to both dentists and medical practitioners.
Some of the significant changes include:
- An expanded guide to the common causes and appropriate management of acute dental pain;
- Clarification of the indications for antibiotic therapy;
- Addition of photos to assist recognition of common disease of the oral mucosa;
- Addition of information relating to appropriate treatment modification for patients with complex medical conditions;
- Expansion of information on medication-related osteonecrosis of the jaw to assist risk assessment and the consequent management of at-risk patients;
- Inclusion of advice for the management of patients at risk of adrenocortical insufficiency;
- Addition of a table summarising the properties of different local anaesthetics along with example calculations of the maximum dose;
- Expanded information on the safe use of anxiolysis in the perioperative period. A printable patient information sheet has also been included;
- Addition of a new section involving the management of peri-implant disease; and
- Expansion of information to assist medical practitioners in the management of common oral and dental conditions.
If you are currently using an offline form of the Therapeutic Guidelines, it is recommended to download the updated version at your earliest convenience.
New contraindications have been added to the product information of Ganfort® (bimatoprost + timolol). It is now advised that these eye drops not be used in patients with sino-atrial nodal block or second or third-degree atrioventricular block not controlled with a pacemaker.
Timolol is a non-selective beta-blocker. Systemic effects of timolol may include bradycardia, orthostatic hypotension, syncope, and bronchoconstriction. While it may sometimes be assumed that eye drops do not produce significant systemic effects, studies suggest that at least 80% of an ophthalmic product drains through the nasolacrimal canal. From there, it may enter the systemic circulation while also avoiding first-pass metabolism.
The current contraindications for Ganfort® eye drops include:
- Bronchospasm;
- Bronchial asthma;
- Severe chronic obstructive pulmonary disease;
- Sinus bradycardia;
- Sick sinus syndrome;
- Sino-atrial nodal block;
- Second or third-degree atrioventricular block not controlled with a pacemaker;
- Overt cardiac failure; and
- Cardiogenic shock.
To avoid systemic absorption, it is recommended to immediately apply pressure to the tear duct for at least two minutes following administration of the eye drop. This is good practice for all eye drops to minimise systemic effects, avoid an unpleasant taste in the mouth, and improve retention at the site of action.
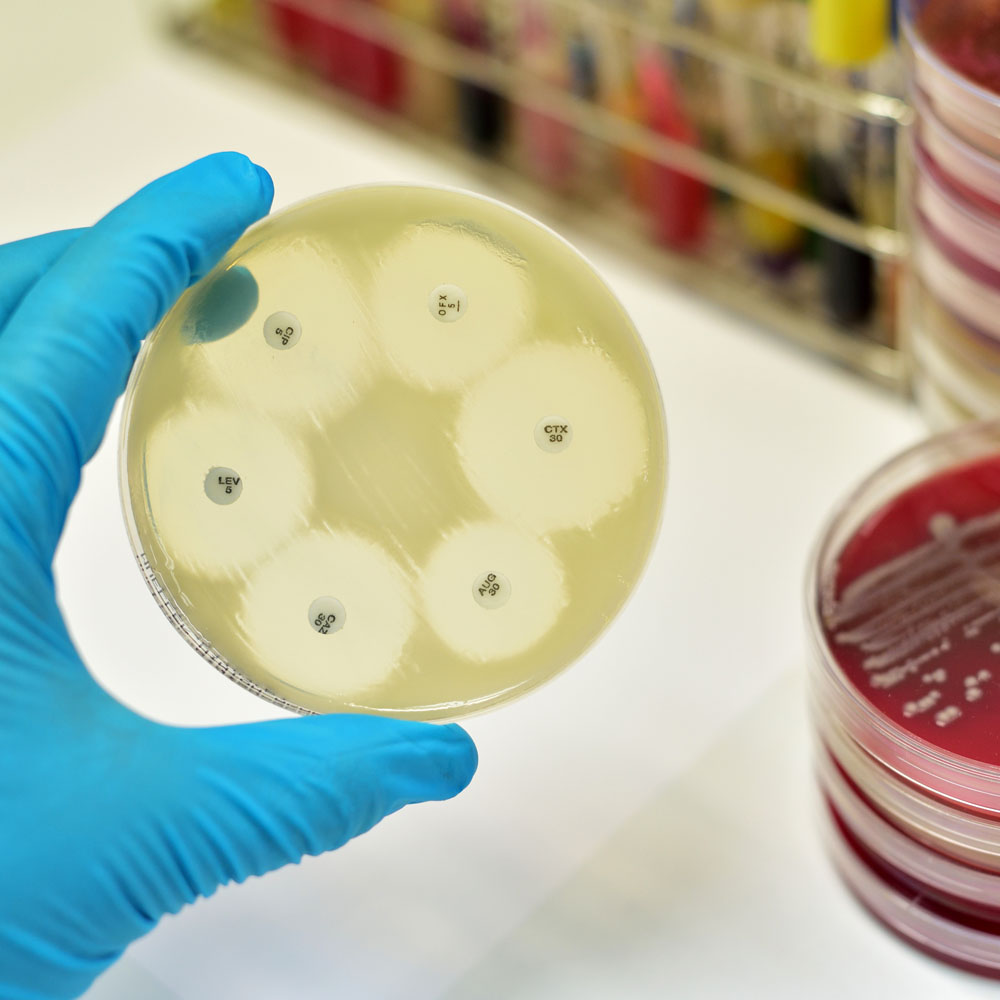
Zavicefta® is a new injectable cephalosporin formulation available in Australia. This product combines the broad-spectrum antibiotic, ceftazidime with the β-lactamase inhibitor, avibactam.
Ceftazidime is one of a limited range of antibiotics with antipseudomonal activity. However, the 2019 AURA Report (Antimicrobial Use and Resistance in Australia) demonstrates that resistance amongst Pseudomonas aeruginosa to ceftazidime has increased from 4.4% of isolates in 2014 to 5.1% in 2017. Avibactam inhibits many of the bacterial enzymes known to inactivate ceftazidime. This extends its spectrum of activity to cover a variety of multi-drug resistant Gram-negative bacteria. However, the formulation has little or no activity against most Gram-positive organisms and anaerobes. Additional agents should be used when such pathogens are known or suspected to be contributing to the infectious process.
Zavicefta® is indicated for the treatment of complicated intra-abdominal infection (in combination with metronidazole), complicated urinary tract infection, and hospital-acquired pneumonia. Ceftazidime and avibactam are both excreted into the urine unchanged; dose adjustment is recommended in moderate and severe renal impairment. The most commonly reported adverse effects are nausea and diarrhoea, which is usually mild to moderate in severity. Clostridium difficile-associated diarrhoea has been reported and should be considered if diarrhoea occurs during, or several weeks after finishing, therapy.
Broad-spectrum antibiotics such as ceftazidime increase the risk of colonisation with antibiotic-resistant microorganisms. They should, therefore, be used judiciously.


Teduglutide is a glucagon-like peptide (GLP) analogue recently registered in Australia for the treatment of short bowel syndrome. There are a number of other GLP analogues available including dulaglutide, exenatide, and liraglutide. While these agents are GLP-1 analogues, teduglutide is an analogue of GLP-2.
The glucagon-like peptides, GLP-1 and GLP-2, are naturally secreted in equivalent amounts but can have very different effects in the body. For example, GLP-1 significantly reduces the levels of the lipoprotein chylomicron, while GLP-2 increases its levels. Other effects of GLP-1 include the promotion of glucose-dependent insulin secretion, preservation of pancreatic β-cell function, slowing of gastric emptying, and reduced appetite. These agents have, therefore, been used in the management of obesity and type 2 diabetes. On the other hand, GLP-2 increases intestinal and portal blood flow, inhibits gastric acid secretion, and slows intestinal motility. These effects have led to the use of teduglutide in the treatment of short bowel syndrome in patients who are dependent on parenteral support.
Clinical studies demonstrate that teduglutide promotes normal growth and repair throughout the intestines. Citrulline, an amino acid produced predominantly by enterocytes, can be considered a marker of enterocyte mass. Over a 24-week study, teduglutide increased plasma citrulline by 20.6µmol/L compared to a 0.7µmol/L increase in the placebo group. Patients in the teduglutide group enjoyed a mean reduction in weekly parenteral support of 4.4L compared to 2.3L in the placebo group.
Adverse effects most commonly reported in clinical trials include abdominal pain and distension, nausea, and injection site reactions. Owing to its pharmacological activity, it is thought that teduglutide may be able to produce hyperplastic changes in the small bowel and hepatobiliary tract. Although this has not been demonstrated in clinical studies, patients should be monitored and therapy discontinued in cases of active gastrointestinal malignancy.
Neratinib is a new tyrosine kinase inhibitor for the treatment of breast cancer. It is indicated as extended adjuvant treatment of early-stage human epidermal growth factor receptor (HER) 2 -positive disease. Initiation is recommended to occur within 12 months of completing trastuzumab therapy.
Tyrosine kinases are important proteins involved in cellular signalling and have a range of biological actions. Members of the HER family of tyrosine kinases promote tumour cell proliferation and survival. Neratinib is known as a pan-HER inhibitor as it has activity at HER1, HER2, and HER4 receptors. Inhibition of these receptors prevents activation of the signal transduction pathway, leading to apoptosis and reduced cellular proliferation. Unlike other tyrosine kinase inhibitors, such as lapatinib, neratinib binds to its target irreversibly.
Diarrhoea is a very common adverse effect, reported in 93.6% of patients. It is recommended that anti-diarrhoeal prophylaxis be initiated with the first dose and continue during the first two months of treatment. Neratinib dose adjustment may be required with permanent discontinuation recommended in severe cases.
Neratinib has a number of clinically important drug interactions. As it is primarily metabolised via cytochrome P450 3A4, exposure can be significantly affected by inducers or inhibitors of this hepatic enzyme. It is contraindicated with strong inducers (e.g. carbamazepine, phenytoin, St John’s wort, and rifampicin) and moderate inhibitors (e.g. fluconazole, diltiazem, verapamil, and erythromycin). Drugs that alter the pH of the upper gastrointestinal tract may also affect therapy as neratinib requires an acidic environment for absorption.

World Antibiotic Awareness Week runs from 18th to 24th November 2019. This annual event aims to promote awareness of antibiotic resistance and improve the use of antibiotics in order to minimise the emergence and spread of antibiotic resistance.
Antimicrobial resistance is considered a global threat to public health. The Antimicrobial Use and Resistance in Australia (AURA) Surveillance System is designed to support Australia’s strategic response to this challenge. The 2019 AURA Report provides an overview of antibiotic use and resistance patterns in Australian hospitals, aged care, general practice, and the community. This report demonstrates that the national rates of resistance for many of the 13 priority organisms have not changed significantly since previous reports. However, several notable exceptions were highlighted, including:
- Increasing resistance of Escherichia coli to common treatment agents;
- Significantly increased resistance of Neisseria gonorrhoeae to azithromycin;
- Very high resistance to ciprofloxacin in typhoidal species of Salmonella; and
- The continued evolution of methicillin resistance patterns in Staphylococcus aureus.
Conclusions from the recent AURA Report indicate that a renewed focus on improving antibiotic use in primary care is required. The World Health Organization (WHO) also emphasises the importance of infection prevention in the fight against antimicrobial resistance. Infection prevention strategies highlighted by the WHO include education and encouragement of proper hand hygiene, vaccination, safe sex practices, and maintenance of good oral hygiene.
Healthcare professionals can refer to Standard 3 of the National Safety and Quality Health Service (NSQHS) Standards for further advice on the prevention of infection and limiting antimicrobial resistance in the healthcare setting.


Buprenorphine is now available as a depot injection for the maintenance treatment of opioid dependence. Two products are registered in Australia, Buvidal® and Sublocade®. However, only Buvidal® is currently accessible on the Pharmaceutical Benefits Scheme (PBS) having received a Section 100 Opioid Dependence listing on the 1st September 2019.
Buvidal® is a modified-release buprenorphine injection for weekly (±2 days) or monthly (±1 week) administration. Upon contact with body fluids, the solution forms a highly viscous gel from which the active ingredient is released in a slow and consistent manner. Buvidal® should be injected slowly into the subcutaneous tissue, taking care to avoid inadvertent intravascular or intradermal injection. Patients may transition directly to Buvidal® from sublingual buprenorphine formulations. However, as the bioavailability of the depot is up to nine times higher than sublingual formulations, appropriate dosage adjustment is required.
A double-blind, double-dummy randomised clinical trial demonstrates non-inferiority of weekly and monthly depot buprenorphine compared to daily sublingual buprenorphine-naloxone. Further analysis establishes the superiority of depot buprenorphine with 35% of urine samples from these patients testing negative for illicit opioids compared to 28% of samples from patients receiving sublingual buprenorphine-naloxone. The safety profile of depot buprenorphine was comparable to that of sublingual buprenorphine-naloxone with the exception of some mild to moderate injection site reactions.
Anticipated benefits of depot buprenorphine injections include:
- Improved adherence;
- Greater convenience;
- Reduced stigma associated with supervised dosing;
- Reduced risk of diversion; and
- Reduced risk of inadvertent medication exposure (e.g. to children from takeaway doses).


The Therapeutic Goods Administration (TGA) has added a boxed warning to febuxostat tablets advising of an increased risk of death in patients with established cardiovascular disease. The addition of this warning follows the publication of the CARES (Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout) trial.
This double-blind non-inferiority study followed over 6,000 patients with coexisting gout and cardiovascular disease. Patients were randomly assigned to receive either febuxostat or allopurinol. A primary end point event (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or unstable angina with urgent revascularisation) occurred at similar rates in the febuxostat group and allopurinol group (7.8% and 7.7% respectively). However, the rate of cardiovascular death was higher in the febuxostat group compared to the allopurinol group (4.3% vs 3.2%), as was the rate of death from any cause (7.8% vs 6.4%). While there is currently no proposed mechanism for this apparent increase in all-cause mortality, the manufacturer advises against using febuxostat in patients with pre-existing major cardiovascular disease. Limitations of this study include high overall rates of premature discontinuation (56.6%) and loss to follow-up (45.0%).
While gout is associated with an increased risk of cardiovascular disease, some evidence suggests that allopurinol may offer cardiovascular benefits. The ongoing ALL-HEART (Allopurinol and Cardiovascular Outcomes in Patients With Ischemic Heart Disease) study is investigating whether allopurinol is associated with improved cardiovascular outcomes in patients with ischaemic heart disease. The results of this trial are expected after 2019.
A naloxone nasal spray is now available for the emergency treatment of known or suspected opioid overdose. Nyxoid® is a single-dose nasal spray containing 1.8mg of naloxone in a 0.1mL solution. Naloxone is an opioid antagonist that can reverse the respiratory and central nervous system depression produced by opioid agonists.
The intranasal route is an attractive one for naloxone. It is rapidly absorbed, allows administration by non-healthcare professionals in emergencies, and avoids issues concerning needlestick injuries and potentially poor intravenous access. However, Nyxoid® is not intended to be a substitute for emergency medical care and does not replace intravenous injection.
The mean half-life of naloxone following intranasal administration is 1.4 hours, considerably less than many opioids. Therefore, repeat doses may be required. In addition, standard naloxone doses may not completely reverse the respiratory depression produced by partial agonists such as buprenorphine. A naloxone infusion may be necessary after the initial intranasal dose, particularly if a long-acting opioid or slow-release formulation is implicated in the overdose event.
Nyxoid® is a Schedule 3 medication and may, therefore, be purchased without a prescription. It is recommended to be carried by people at risk of, or likely to witness opioid overdose events. The indications, instructions for use and requirement for additional emergency medical care must be clearly explained to any person who may need to administer this product. The Penington Institute provides educational materials appropriate for non-healthcare professionals.




















