HPS Pharmacies wish to advise that Pfizer is experiencing a supply interruption for heparin as follows:
Heparin Sodium
Heparin sodium 25,000IU/5mL
ARTG 49236
This supply interruption is expected to be resolved by the end of December 2024.
An internationally registered alternative has been approved for supply under Section 19A of the Therapeutic Goods Act 1989. The alternative product is also preservative-free, and all packaging is in English.
Heparin is a high-risk medication. Additional care is recommended when selecting products, particularly when the packaging may be unfamiliar. The following table compares the Australian-registered product with the S19A alternative.
| |
Heparin sodium 25000IU/5mL (Pfizer) |
Heparin sodium (Wockhardt) preservative-free |
| Product type |
Australian registered |
S19A |
| Excipients |
Water for injection |
- Water for injections
- Sodium hydroxide solution 3M
- Hydrochloric acid 3M
|
| Pack size |
50 Steriluer ampoules |
10 neutral glass ampoules |
| Storage |
< 25°C |
Do not store > 25°C
Store in original package |
| Additional information |
Single-use only |
Single-use only |
Retain this notice in a prominent position, including in other related business units until supplies return to normal. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Pfizer on 1800 675 229 or your pharmacist at HPS Pharmacies.
Download PDF

HPS Pharmacies wish to advise that all sponsors are currently experiencing a supply interruption for ezetimibe 10mg tablets.
Normal supplies are expected to return in mid-October 2024 for some brands, although other brands are not expected to return until mid-November 2024.
An internationally registered alternative has been approved for supply under Section 19A of the Therapeutic Goods Act 1989. This product is registered for use in the United States and all packaging is in English.
Some ezetimibe combination products may also be affected by supply issues during this period.
Retain this notice in a prominent position, including in other related business units, until supplies return to normal. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact your pharmacist at HPS Pharmacies.
Download PDF

HPS Pharmacies wish to give notice that Baxter, in consultation with the Therapeutic Goods Administration (TGA), has initiated a product defect correction for Compounded Parenteral Nutrition and IV Solutions.
For a full list of affected products, please click here.
This product defect correction has been initiated following increased reports of particulate matter in disposable inlets used with the ExactaMix (EM2400) Automated Compounding device used by Baxter Compounding for preparation of selected Parenteral Nutrition and IV Solutions. There is a risk that particulate matter may be present in final compounded solutions. There is no risk in relation to sterility of the tubing.
Baxter is working to replace the inlets affected by this issue. Until replacement stock is available, Baxter Compounding has ensured additional measures are in place to minimise the risk of particulates in compounded solutions, including:
- Inspecting the inlets prior to use in compounding;
- Priming the inlets prior to use; and
- Performing Visual Inspection on each filled compounded unit
Baxter also advises healthcare professionals to:
- Inspect and discard/return any product with visible particles; and
- Use an appropriate in-line filter set during the administration of any compounded solutions manufactured using the affected inlets. An in-line filter of not less than 1.2 micron should be used for administration of parenteral nutrition containing a lipid, a 0.22 micron filter can be used for aqueous solutions without a lipid (in line with international guidelines).
Please inspect your stock and quarantine any defect stock. If HPS Pharmacies provide imprest management services to your facility, an HPS representative will inspect all stock stored in the designated imprest areas at the earliest opportunity. All affected products will be quarantined, and HPS Pharmacies will arrange for a credit as soon as possible. An HPS Pharmacies staff member will contact senior medical and nursing staff if there is an immediate impact to your facility.
Retain this notice in a prominent position, including in other related business units for one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Baxter on 1800 229 837 or your pharmacist at HPS Pharmacies.
Download PDF

HPS Pharmacies wish to advise that Orion is experiencing a supply interruption for Bisalax® as follows:
Bisalax® Enema
Bisacodyl 10mg/5mL
ARTG 27900
This supply interruption is expected to resolve by early November 2024.
An internationally registered alternative has been approved for supply under Section 19A of the Therapeutic Goods Act 1989. This product, Toilax®, is registered for use in Finland and all packaging is in Finnish and Swedish. However, the active ingredient and strength are recognisable in English.
The Finnish product is manufactured in the same facility as the Australian registered product and is identical for active ingredient and strength. The differences between the two products are shown in the table below.
| |
Bisalax® |
Toilax® |
| Product type |
Australian registered |
S19A |
| Labelled strength |
10mg/5mL |
2mg/mL |
| Pack size |
25 enema tubes |
50 enema tubes |
| Language |
English |
Finnish and Swedish |
As the patient leaflet contained within the S19A alternative is not in English, patients can be referred to the Australian Consumer Medicines Information (CMI) brochure.
Retain this notice in a prominent position, including in other related business units, until supplies return to normal. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Orion on (02) 8067 8704 or your pharmacist at HPS Pharmacies.
Download PDF

HPS Pharmacies wish to give notice that AbbVie, in consultation with the Therapeutic Goods Administration (TGA), has initiated a drug recall for Refresh® Night Time Eye Ointment as follows:
Refresh® Night Time Eye Ointment (2×3.5g)
Eye lubricant
ARTG 370296
The only batch affected by this recall is: 397925 (Expiry: Oct 2024)
This device recall was initiated as products in the affected batch may contain a breach in the tube seal.
Please inspect your stock and quarantine any defect stock. If HPS Pharmacies provide imprest management services to your facility, an HPS representative will inspect all stock stored in the designated imprest areas at the earliest opportunity. All affected products will be quarantined, and HPS Pharmacies will arrange for a credit as soon as possible. An HPS Pharmacies staff member will contact senior medical and nursing staff if there is an immediate impact to your facility.
There is currently no replacement stock available for Refresh® Night Time Eye Ointment. However, many other brands of eye lubricant products are available for consideration.
Retain this notice in a prominent position, including in other related business units for one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact AbbVie on 1800 252 224 or your pharmacist at HPS Pharmacies.
Download PDF

HPS Pharmacies wish to advise that Bayer is experiencing a supply interruption for Urografin® as follows:
Urografin® 30% 250mL
Sodium amidotrizoate / amidotrizoate meglumine
ARTG 42163
Supplies are expected to return to normal by the end of December 2024.
Internationally registered alternatives may be accessed via the Special Access Scheme (SAS). The lead time for accessing SAS stock is longer, and pricing will vary.
Retain this notice in a prominent position, including in other related business units until supplies return to normal. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Bayer Radiology on 1800 039 076 or your pharmacist at HPS Pharmacies.
Download PDF

HPS Pharmacies wish to advise that Bridgewest is experiencing a supply interruption for atropine ampoules as follows:
Bridgewest Atropine Injection BP
Atropine sulfate monohydrate 600 microgram/1 mL
ARTG 11302
Bridgewest Atropine Injection BP
Atropine sulfate monohydrate 1200 microgram/1 mL
ARTG 11303
This supply interruption is expected to resolve by early October 2024. Some warehouses may still have supplies of atropine 600 microgram/1mL.
Atropine Juno 600 microgram/1mL has been discontinued by the sponsor.
Internationally registered alternatives may be accessed via the Special Access Scheme (SAS). The lead time for accessing SAS stock is longer, and pricing will vary.
Retain this notice in a prominent position, including in other related business units until supplies return to normal. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Bridgewest on 1800 161 156 or your pharmacist at HPS Pharmacies.
Download PDF
HPS Pharmacies wishes to advise that Novo Nordisk is discontinuing the following insulin products:
Mixtard® 30/70 InnoLet®, 3mL
Insulin neutral human 30 units/mL + Insulin isophane human 70 units/mL
ARTG 169628
Mixtard® 30/70 Penfill®, 3mL
Insulin neutral human 30 units/mL + Insulin isophane human 70 units/mL
ARTG 169629
Fiasp® Vials, 10mL
Insulin aspart (rys) 100 units/mL
ARTG 275375
Fiasp® Flextouch Pens, 3mL
Insulin aspart (rys) 100 units/mL
ARTG 275394
The sponsor has already discontinued Mixtard® 30/70 InnoLet®, although warehouses may still have some remaining stock. Mixtard® 30/70 Penfill® is due to be deleted from the market in October 2024. Once warehouse supplies have been exhausted, no other presentation of Mixtard® 30/70 will be available. Humulin® 30/70 is an alternative brand that remains available in a 10mL vial and 3mL cartridge.
Fiasp® vials are expected to be available until December 2024; Fiasp® Flextouch pre-filled pens are expected to be available until June 2025. Fiasp® will remain available in the Penfill presentation only.
Retain this notice in a prominent position, including in other related business units for at least one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Novo Nordisk on 1800 668 626 or your pharmacist at HPS Pharmacies.
Download PDF

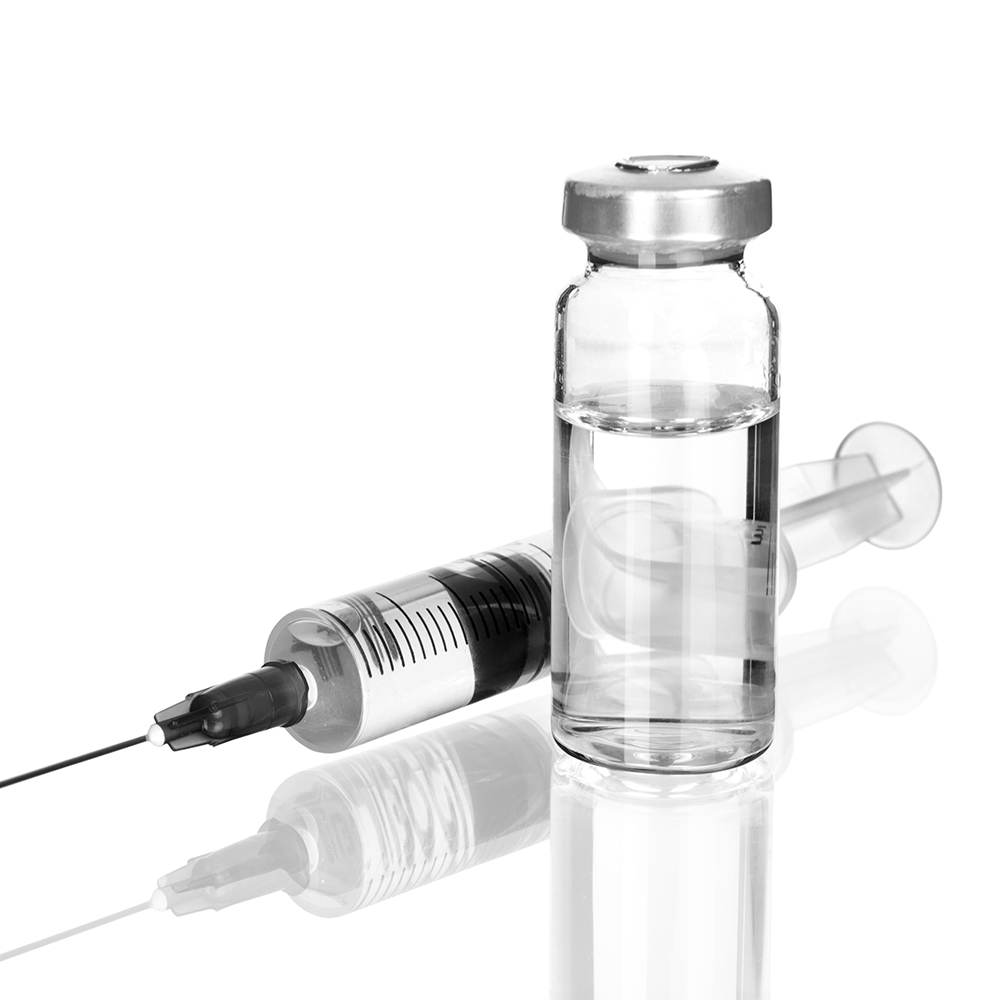
HPS Pharmacies wish to advise that Pfizer is experiencing a supply interruption for Heparinised Saline as follows:
Heparinised Saline
Heparin sodium 50 IU/5mL
ARTG 66684
This supply interruption is expected to resolve by the end of January 2025.
An internationally registered alternative has been approved for supply under Section 19A of the Therapeutic Goods Act 1989. This product is registered for use in the United Kingdom and all packaging is in English. It is identical to the Australian registered product for active ingredient, strength, and excipients. However, there are some presentation differences, as shown in the table below.
| |
Heparinised saline (Pfizer) |
Heparin sodium flushing solution (Wockhardt) |
| Product type |
Australian registered |
S19A |
| Labelled as |
Heparinised saline |
Heparin sodium flushing solution |
| Pack size |
50 ampoules |
10 ampoules |
| Presentation |
LDPE ampoules |
Glass ampoules |
The S19A alternative (Heparin sodium flushing solution [Wockhardt]) is pictured below. The Summary of Product Characteristics can be accessed here.
Retain this notice in a prominent position, including in other related business units until supplies return to normal. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Pfizer on 1800 675 229 or your pharmacist at HPS Pharmacies.
Download PDF

HPS Pharmacies wish to advise that Pfizer is experiencing a supply interruption for heparin as follows:
Heparin Sodium
Heparin sodium 5000IU/5mL
ARTG 49232
This supply interruption is expected to be resolved by end of December 2024.
Internationally registered alternatives have been approved for supply under Section 19A of the Therapeutic Goods Act 1989. Please note that some products approved under S19A contain benzyl alcohol as a preservative, while the Australian-registered product is preservative-free. A preservative-free option is currently approved for supply under S19A until 30 September 2024.
Benzyl alcohol should not be used in neonates or infants and should be avoided during pregnancy. Clinical review may also be required in other patient groups, depending on the duration of use.
Please note that S19A alternatives are subject to a longer lead time and different pricing as they must be sourced internationally.
Heparin is a high-risk medication. Additional care is recommended when selecting products, particularly when the packaging may be unfamiliar. The following table compares the Australian-registered product with the S19A alternatives.
| |
Heparin sodium 5000IU/5mL (Pfizer) |
Heparin sodium injection, USP 10,000 units/10 mL (Hospira) |
Heparin sodium (Wockhardt) – with preservative |
Heparin sodium (Wockhardt) preservative-free |
| Product type |
Australian registered |
S19A |
S19A |
S19A |
| Labelled strength |
5000IU/5mL |
10,000 USP units/10 mL (1,000 USP units/mL) |
1,000 I.U./mL (5,000 units in 5 mL) |
1,000 I.U./mL (5,000 units in 5 mL) |
| Excipients |
Water for injection |
Sodium chloride
Benzyl alcohol
Sodium hydroxide
Hydrochloric acid
Water for injections |
Benzyl alcohol
Methyl parahydroxybenzoate
Water for injections
Sodium hydroxide solution
Hydrochloric acid |
Water for injections
Sodium hydroxide solution
Hydrochloric acid |
| Administration route |
IV or SC |
IV or SC |
IV |
IV |
| Presentation |
5 mL steriluer ampoule |
10 mL glass vial |
5 mL neutral glass vial |
5 mL glass ampoule |
| Storage |
< 25°C |
20°C to 25°C |
Do not store > 25°C |
Do not store > 25°C |
| Additional information |
Single-use only |
Multidose vial (use in line with hospital policy). |
Multidose vial (use in line with hospital policy). |
Single-use only |
*Note: One USP unit is equivalent to one international unit (IU)
Retain this notice in a prominent position, including in other related business units until supplies return to normal. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Further information from Pfizer is available here (note: Pfizer has since revised the estimated return date to December 2024). Should you require further information regarding this matter, please contact Pfizer on 1800 675 229 or your pharmacist at HPS Pharmacies.
Download PDF