
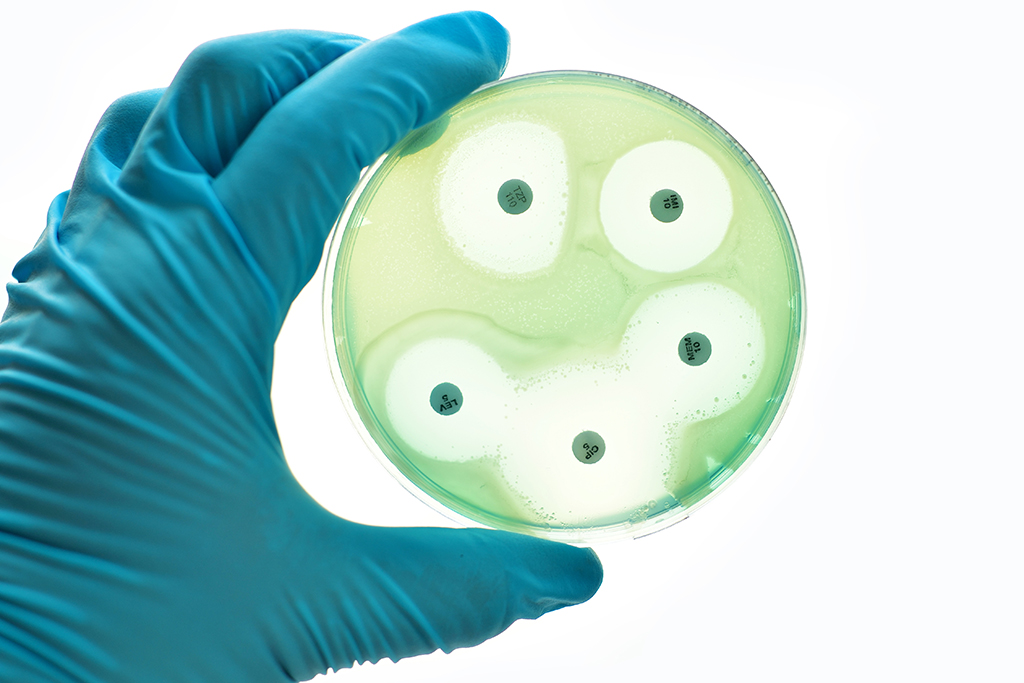
World Antibiotic Awareness Week runs from 18th to 24th November 2019. This annual event aims to promote awareness of antibiotic resistance and improve the use of antibiotics in order to minimise the emergence and spread of antibiotic resistance.
Antimicrobial resistance is considered a global threat to public health. The Antimicrobial Use and Resistance in Australia (AURA) Surveillance System is designed to support Australia’s strategic response to this challenge. The 2019 AURA Report provides an overview of antibiotic use and resistance patterns in Australian hospitals, aged care, general practice, and the community. This report demonstrates that the national rates of resistance for many of the 13 priority organisms have not changed significantly since previous reports. However, several notable exceptions were highlighted, including:
- Increasing resistance of Escherichia coli to common treatment agents;
- Significantly increased resistance of Neisseria gonorrhoeae to azithromycin;
- Very high resistance to ciprofloxacin in typhoidal species of Salmonella; and
- The continued evolution of methicillin resistance patterns in Staphylococcus aureus.
Conclusions from the recent AURA Report indicate that a renewed focus on improving antibiotic use in primary care is required. The World Health Organization (WHO) also emphasises the importance of infection prevention in the fight against antimicrobial resistance. Infection prevention strategies highlighted by the WHO include education and encouragement of proper hand hygiene, vaccination, safe sex practices, and maintenance of good oral hygiene.
Healthcare professionals can refer to Standard 3 of the National Safety and Quality Health Service (NSQHS) Standards for further advice on the prevention of infection and limiting antimicrobial resistance in the healthcare setting.


Buprenorphine is now available as a depot injection for the maintenance treatment of opioid dependence. Two products are registered in Australia, Buvidal® and Sublocade®. However, only Buvidal® is currently accessible on the Pharmaceutical Benefits Scheme (PBS) having received a Section 100 Opioid Dependence listing on the 1st September 2019.
Buvidal® is a modified-release buprenorphine injection for weekly (±2 days) or monthly (±1 week) administration. Upon contact with body fluids, the solution forms a highly viscous gel from which the active ingredient is released in a slow and consistent manner. Buvidal® should be injected slowly into the subcutaneous tissue, taking care to avoid inadvertent intravascular or intradermal injection. Patients may transition directly to Buvidal® from sublingual buprenorphine formulations. However, as the bioavailability of the depot is up to nine times higher than sublingual formulations, appropriate dosage adjustment is required.
A double-blind, double-dummy randomised clinical trial demonstrates non-inferiority of weekly and monthly depot buprenorphine compared to daily sublingual buprenorphine-naloxone. Further analysis establishes the superiority of depot buprenorphine with 35% of urine samples from these patients testing negative for illicit opioids compared to 28% of samples from patients receiving sublingual buprenorphine-naloxone. The safety profile of depot buprenorphine was comparable to that of sublingual buprenorphine-naloxone with the exception of some mild to moderate injection site reactions.
Anticipated benefits of depot buprenorphine injections include:
- Improved adherence;
- Greater convenience;
- Reduced stigma associated with supervised dosing;
- Reduced risk of diversion; and
- Reduced risk of inadvertent medication exposure (e.g. to children from takeaway doses).


The Therapeutic Goods Administration (TGA) has added a boxed warning to febuxostat tablets advising of an increased risk of death in patients with established cardiovascular disease. The addition of this warning follows the publication of the CARES (Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout) trial.
This double-blind non-inferiority study followed over 6,000 patients with coexisting gout and cardiovascular disease. Patients were randomly assigned to receive either febuxostat or allopurinol. A primary end point event (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or unstable angina with urgent revascularisation) occurred at similar rates in the febuxostat group and allopurinol group (7.8% and 7.7% respectively). However, the rate of cardiovascular death was higher in the febuxostat group compared to the allopurinol group (4.3% vs 3.2%), as was the rate of death from any cause (7.8% vs 6.4%). While there is currently no proposed mechanism for this apparent increase in all-cause mortality, the manufacturer advises against using febuxostat in patients with pre-existing major cardiovascular disease. Limitations of this study include high overall rates of premature discontinuation (56.6%) and loss to follow-up (45.0%).
While gout is associated with an increased risk of cardiovascular disease, some evidence suggests that allopurinol may offer cardiovascular benefits. The ongoing ALL-HEART (Allopurinol and Cardiovascular Outcomes in Patients With Ischemic Heart Disease) study is investigating whether allopurinol is associated with improved cardiovascular outcomes in patients with ischaemic heart disease. The results of this trial are expected after 2019.
A naloxone nasal spray is now available for the emergency treatment of known or suspected opioid overdose. Nyxoid® is a single-dose nasal spray containing 1.8mg of naloxone in a 0.1mL solution. Naloxone is an opioid antagonist that can reverse the respiratory and central nervous system depression produced by opioid agonists.
The intranasal route is an attractive one for naloxone. It is rapidly absorbed, allows administration by non-healthcare professionals in emergencies, and avoids issues concerning needlestick injuries and potentially poor intravenous access. However, Nyxoid® is not intended to be a substitute for emergency medical care and does not replace intravenous injection.
The mean half-life of naloxone following intranasal administration is 1.4 hours, considerably less than many opioids. Therefore, repeat doses may be required. In addition, standard naloxone doses may not completely reverse the respiratory depression produced by partial agonists such as buprenorphine. A naloxone infusion may be necessary after the initial intranasal dose, particularly if a long-acting opioid or slow-release formulation is implicated in the overdose event.
Nyxoid® is a Schedule 3 medication and may, therefore, be purchased without a prescription. It is recommended to be carried by people at risk of, or likely to witness opioid overdose events. The indications, instructions for use and requirement for additional emergency medical care must be clearly explained to any person who may need to administer this product. The Penington Institute provides educational materials appropriate for non-healthcare professionals.


The Therapeutic Goods Administration (TGA) is updating product information documents for direct acting oral anticoagulants (DOACs) to advise of the increased risk of recurrent thrombotic events in patients with antiphospholipid syndrome. Antiphospholipid syndrome is an autoimmune disorder in which patients develop antibodies to phospholipid-bound proteins. Patients with this condition are at an increased risk of thrombosis and standard treatment involves the use of anticoagulants.
Anticoagulants include heparin, warfarin, and DOACs. The three DOACs available in Australia are apixaban, dabigatran etexilate, and rivaroxaban. This new warning is based on results from the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) study conducted in patients with antiphospholipid syndrome who have a history of thrombosis and a high risk of recurrent events. This study demonstrates an increased risk of thrombotic events with rivaroxaban compared to warfarin. Thromboembolic events occurred in 21% of patients in the rivaroxaban group compared to 0% for patients in the warfarin group. Major bleeding events were reported in 7% of patients in the rivaroxaban and 3% in the warfarin group. While the study only investigated rivaroxaban, other DOACs may be associated with a similar risk.
The TGA advises that DOACs are not recommended for patients with antiphospholipid syndrome, particularly those who are considered high-risk. If these patients are currently receiving a DOAC, it may be appropriate to consider changing to an alternative anticoagulant.
Painful conditions of the mouth are uncomfortable and can significantly impair oral intake of fluid and nutrition. Mucositis is often cited as the most debilitating side effect of cancer chemotherapy and radiotherapy. Options recommended by eviQ for the symptomatic management of oral mucositis and stomatitis include systemic analgesics, topical anti-inflammatories, topical steroids, and local anaesthetic mouthwashes.
When it comes to local anaesthetic mouthwashes, there are few proprietary products available. Xylocaine® Viscous, containing 2% lidocaine, is one option. This product is formulated as a thick solution to increase adherence to the oral mucosa. Absorption from the gastrointestinal tract is relatively high, although systemic lidocaine exposure is usually low due to extensive first-pass metabolism. Caution is advised in patients with hepatic impairment as the half-life may be increased up to three-fold. While true allergic reactions to local anaesthetics are rare, Xylocaine® Viscous is contraindicated in patients with a known history of hypersensitivity to lidocaine or any other amide-type local anaesthetic.
Cocaine may be considered for patients who are allergic to amide local anaesthetics. Cocaine belongs to the ester family of local anaesthetics and does not display cross-reactivity with amides. No cocaine products are currently registered with the Therapeutic Goods Administration (TGA). However, cocaine mouthwash is produced by a TGA-licensed manufacturer and is now available in 0.5% (200mL) and 1% (100mL) strengths.
Cocaine is primarily metabolised in the plasma and tissue fluids. Its anaesthetic effect is similar to other local anaesthetics, although it does possess some unique characteristics. It is a vasoconstrictor across all dosing levels and induces platelet activation and thrombus formation. These effects may be useful to prolong the duration of effect, minimise systemic absorption, reduce swelling, and improve haemostasis in patients with mucosal bleeding.
There is a risk of systemic toxicity if used in patients with traumatised mucosa or if the recommended dose is exceeded. The estimated LD50 (lethal dose that is fatal in 50% of cases) for cocaine is 500mg for adults following oral administration. This equates to 50mL of the 1.0% solution. It is, therefore, crucial to stress the importance of adhering to dosing recommendations and to expectorate the solution following use. Caution is advised in patients with hypertension, cardiovascular disease, thyroid disease, severe hepatic impairment, or a history of drug abuse.

Rufinamide is a new adjunctive therapy in the treatment of seizures associated with Lennox-Gastaut syndrome (LGS). LGS is a severe form of epilepsy that presents with multiple types of seizures and is often refractory to treatment. A double-blind, randomised, placebo-controlled trial conducted in patients with LGS demonstrated a 42.5% reduction in tonic-atonic seizures in the rufinamide-treated group compared to a 1.4% increase in the placebo group. A number of other clinical trials demonstrate favourable efficacy in reducing the frequency and severity of various seizure types.
The most commonly reported adverse effects include headache, dizziness, fatigue, and somnolence. Some clinical studies have reported status epilepticus occurring in rufinamide-treated patients but not in the placebo groups. While status epilepticus is reasonably common in a population with refractory epilepsy, rufinamide therapy should be reviewed if patients experience new or increasing frequency of seizures.
Rufinamide is not metabolised by cytochrome (CYP) P450 enzymes and does not inhibit their activity to any significant extent. It is a weak inducer of CYP3A4 and may reduce the plasma concentrations of CYP3A4 substrates. Significant increases in plasma rufinamide levels can occur when valproate is initiated while rufinamide levels may be reduced by phenytoin, phenobarbital, and primidone. The absorption of rufinamide is significantly affected by food. Bioavailability and peak plasma concentrations rise by 34% and 56% respectively when administered with meals. It is advised that doses are taken with food.


Telotristat is a new medication for the treatment of carcinoid syndrome diarrhoea. Carcinoid syndrome is a condition that develops in around 10% of people with a carcinoid tumour. Carcinoid tumours develop from enterochromaffin cells, the most common type of neuroendocrine cell in the gastrointestinal tract. Tumours derived from these cells can produce large amounts of vasoactive substances, including serotonin and histamine. This may contribute to symptoms of carcinoid syndrome such as severe diarrhoea, bronchoconstriction, flushing, and abdominal cramps.
Telotristat inhibits peripheral tryptophan hydroxylase, an enzyme involved in the biosynthesis of serotonin. Consequently, serotonin levels decrease and symptoms of carcinoid syndrome are alleviated. Telotristat is designed not to cross the blood-brain barrier, and studies demonstrate no significant change in brain serotonin levels at the recommended dose. However, patients should be advised to report any symptoms of depression or altered mood to their doctor.
Telotristat is an oral medication that should be taken with food. It is indicated for use in combination with a somatostatin analogue such as octreotide or lanreotide. Short-acting octreotide causes a significant reduction in systemic exposure to telotristat and its active metabolite and should be administered at least 30 minutes after a telotristat dose.

In addition to the discontinuation of pembrolizumab 50mg vials, Merck Sharp & Dohme have advised of some upcoming changes to the Pharmaceutical Benefits Scheme (PBS) listing for the 100mg/4mL vials. These changes affect the dosing of pembrolizumab in the treatment of unresectable Stage III or Stage IV malignant melanoma.
From 1st August 2019, the PBS dosing criteria for this indication will be:
- Initial treatment – up to six doses administered every three weeks with each maximum dose fixed at 200mg; and
- Continuing treatment – maximum dose administered every three weeks fixed at 200mg.
These dosing criteria will replace the current maximum dose of 2mg/kg (up to 240mg). Pembrolizumab was first registered in Australia for the treatment of melanoma. Clinical studies used to support the initial application used weight-based doses of 2mg/kg and 10mg/kg. Later studies demonstrated equal efficacy of the higher and lower weight-based doses, leading to the introduction of a fixed 200mg dose for other indications.
The product information should be referred to for further dosing information and the PBS website for current PBS criteria. The PBS listing for the 50mg presentation of pembrolizumab will be deleted on 1st September 2019.

As discussed in Drugline Volume 354, pembrolizumab is available on the Pharmaceutical Benefits Scheme (PBS) for the first-line treatment of metastatic non-small cell lung cancer.
In the original KEYNOTE-024 trial, the median duration follow up was 11.2 months and pembrolizumab was associated with a significantly longer progression-free survival (10.3 versus 6.0 months) and overall survival at six months (80.2% versus 72.4%) compared to platinum-based chemotherapy.
An updated analysis of KEYNOTE-024 with a median follow up duration of 25.2 months demonstrated pembrolizumab to have a significantly improved overall survival (30 months vs 14.2 months) and a median duration of response not reached.
The 24-month overall survival rate was 51.5% in the pembrolizumab group vs 34.5% in the chemotherapy group.
The incidence of treatment-related adverse effects remains consistent as the interim data, pembrolizumab at 76.6% and chemotherapy at 90%. However, immune-mediated adverse effects including hypo- and hyperthyroidism, pneumonitis, skin toxicity and infusion reactions were markedly higher, with pembrolizumab at 33.8% and chemotherapy at 5.3%.




















