Product Notice
Further to DrugAlert Vol 487, HPS Pharmacies wish to advise that Juno Pharmaceuticals has updated the packaging and labelling for metaraminol syringes as follows:
Metaraminol Juno Syringe
Metaraminol (as tartrate) 5mg/10mL
ARTG 298220 + 298219
This change has occurred in response to concerns of look-alike packaging with adrenaline syringes and saline flush syringes. The labelling on the syringe barrel and the external plastic wrap are now purple (as shown below) in accordance with the anaesthetic labelling standard for vasopressors.
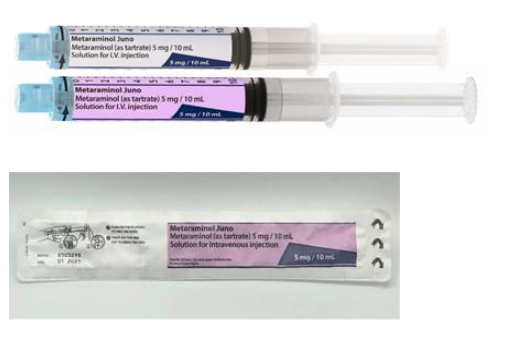
In addition, the new batch has been manufactured using a new barrel mould. Juno Pharmaceuticals is confident that this has rectified any concerns related to leakage. Testing found no issues when used with the following adaptors/IV sets:
- BD SmartSite™;
- BBraun Safesite®;
- BBraun extension line; and
- Tuta connector.
It is expected that the new packaging for Metaraminol Juno Syringes will begin to arrive from wholesalers shortly. It is recommended that additional diligence be exercised to prevent selection errors during this changeover period when products with old and new packaging may be present in clinical areas. There has been no change to the formulation of this product.
Retain this notice in a prominent position, including in other related business units for one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact your pharmacist at HPS Pharmacies, or Juno on (03) 8888 1288.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
