Presentation Change
HPS Pharmacies wish to give notice that Phebra has updated the packaging of their Phenasen® vials as follows:
Phenasen® Vials
Arsenic trioxide 10mg/10mL
ARTG 152760
The packaging changes have been made to assist with the safe handling of arsenic trioxide solution.
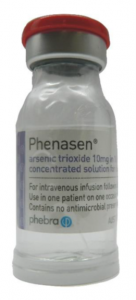
From batch number 14762 and for all subsequent batches, Phenasen® will be supplied with the following features, as shown in Image 1:
- A transparent protective sleeve that completely encapsulates each vial. In the event of a vial breakage, arsenic trioxide solution will be contained within the sheath; and
- A rigid plastic bottom that provides added stability to prevent the vial from tipping, as well as additional impact resistance in the event of accidental dropping.
Image 1. Updated Phenasen® presentation
Retain this notice in a prominent position, including in other related business units for one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Phebra on 1800 720 020, or your pharmacist at HPS Pharmacies.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
