HPS Pharmacies wish to give notice of an alert for Xylocaine® 2% with Adrenaline as follows:
Xylocaine® 2% with Adrenaline Vial, 20mL
Lidocaine (lignocaine) 5mg/mL + adrenaline (epinephrine) 5microgram/mL
This alert only relates to the internationally-registered product supplied under Section 19A of the Therapeutic Goods Act 1989 while the Australian-registered product is unavailable.
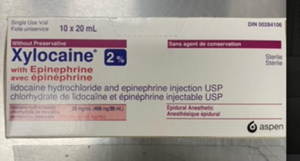
HPS Pharmacies has been advised that the Canadian supplier of the S19A alternative has incorrectly shipped a product containing preservative instead of the preservative-free product. The administration of Xylocaine® with preservative may cause patient harm, particularly when the intrathecal or epidural routes are used.
The only batch affected by this issue is batch #9949864.
HPS Pharmacies can confirm that we have not received or supplied to our clients any product from this batch. The similar packaging of the two products is shown below for informational purposes.
| Xylocaine® 2% with Adrenaline with preservative:
|
Xylocaine® 2% with Adrenaline without preservative:
|
Retain this notice in a prominent position, including in other related business units for one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please refer to the Safer Care Victoria notice (available here) or contact your pharmacist at HPS Pharmacies.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates