Introduction
It is important to distinguish between three commonly used descriptors in connection with the current viral pandemic. These are:
- Coronavirus;
- SARS-CoV-2; and
- COVID-19.
Coronaviruses are a large group of viruses that are the genesis of respiratory infections and can cause mild medical problems such as the common cold through to more serious infections. This group of viruses is so named because of the crown-like spikes on its surface. There are four common human coronaviruses: 229E, NL63, OC43, and HKU1. Common human coronaviruses usually only cause mild to moderate upper-respiratory tract symptoms, such as the common cold. Those who contract these viruses are usually able to recover with only mild supportive medical assistance.
There are three other coronaviruses, which originate from animal sources and transfer to humans:
- SARS-CoV (severe acute respiratory syndrome – coronavirus), which first appeared in southern China in November 2002;
- MERS-CoV (Middle Eastern respiratory syndrome), which emerged in Saudi Arabia in 2012, but may have originated in Jordan; and
- SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2), which originated in Wuhan, China in December 2019.
SARS-CoV-2 is the name given to the 2019 novel coronavirus. It is an enveloped, single-stranded, positive-sense RNA virus and is the cause of COVID-19, the resultant disease. COVID-19 results in death associated with acute respiratory distress syndrome (ARDS), usually as a result of pneumonia.
SARS-CoV-2 had not previously been identified in humans prior to its emergence in China in 2019. It is now understood that SARS2-CoV-2 originated in bats. The capacity for a disease to transfer from an animal to a human is called zoonosis, and the infection is called a zoonotic disease.
Researchers have demonstrated that SARS2-CoV-2 is 88% genetically related to two bat-derived SARS-like coronaviruses, (known as bat-SL-CoVZC45 and bat-SL-CoVZXC21), collected in 2018 in Zhoushan, eastern China. It is more distantly related to SARS-CoV (about 79%) and MERS-CoV (about 50%).
Other researchers have reported “nCoV-2019 is 96% identical at the whole genome level to a bat coronavirus.”
Current possible treatments.
There is considerable current interest in the use of hydroxychloroquine (HCQ) or chloroquine (CQ) as mitigating treatments for COVID-19.
Currently HCQ is used for rheumatoid arthritis and systemic lupus erythematous. It is also used for malaria, but only in areas where chloroquine is effective, such as the Caribbean and Central America. Due to increasing widespread resistance by P. falciparum and in P. vivax in Indonesia and the Pacific, chloroquine is used with diminishing frequency for malaria.
Chloroquine (a) differs from hydroxychloroquine (b) in that the latter has a hydroxyl group at the end of a side-chain. Hydroxychloroquine is a metabolite of chloroquine.19 20
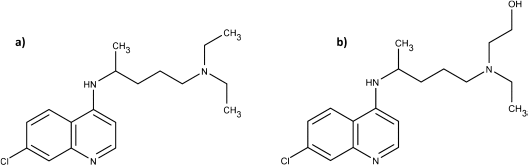
This slight structural change has resulted in less toxicity for HCQ compared to CQ, notably less retinopathy. Rare cases of cardiomyopathy have also been reported with chloroquine.
The biological mechanism of CQ/HCQ.
There are several postulated mechanisms by which CQ/HCQ may act against SARS-CoV-2:
- The virus is believed to enter a cell by binding to angiotensin-converting enzyme 2 (ACE2), which is found on the cell surface. The anti-SARS-CoV-1 action of CQ in vitro is attributed to an antagonist ACE-2 effect, thus impeding viral-cell surface binding.
- CQ has also been shown to increase the endosomal pH, which may impair SARS-CoV early stage viral replication.
CQ/HCQ dosing:
A dose of hydroxychloroquine 600mg per day has been shown to achieve a concentration of 1ug/mL. It is unclear if this is a suitable therapeutic dose.
Gao (2020, Feb 19th) reported that “results from more than 100 patients have demonstrated that chloroquine phosphate is superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus negative conversion, and shortening the disease course…”
An uncorrected manuscript from Huang and co-workers (April 2020) gives a detailed analysis of chloroquine 500mg twice daily for 10 days (n= 10) compared to lopinavir/ritonavir 400/100mg (n=12) twice daily for 10 days as follows:
The first patient achieved lung clearance based on CT imaging was from the Lopinavir/Ritonavir group at Day 6 and this patient became SARS-CoV-2 negative at Day 3.
In the chloroquine group, the first patient achieved lung clearance was at Day 8 who became SARS-CoV-2 negative at Day 7… These data suggest that viral clearance does not translate immediately into pathological improvement in lungs. By Day 9, 6 patients (60%) in the Chloroquine group reached lung clearance, compared to 3 (25%) from the Lopinavir/Ritonavir group…
By Day 14, the incidence rate of lung improvement based on CT imaging from the Chloroquine group was more than double that of the Lopinavir/Ritonavir group (rate ratio 2.21, 95% CI 0.81-6.62).
These results suggest that patients treated with Chloroquine appear to recover better and regain their pulmonary function quicker than those treated with Lopinavir/Ritonavir.
During the chloroquine treatment period, there were nine adverse reactions in five patients including vomiting, abdominal pain, nausea, diarrhoea, rash or itchy, cough and shortness of breath. No serious adverse reactions were reported and there were no patient withdrawals. The serum concentration of chloroquine was in the range of 0.26 – 0.61μmol/L.
T-cell counts were taken for 10 CQ patients every two days. CD3+, CD4+, CD8+ counts showed no significant reduction in T-cell counts during the 10-days of treatment and chloroquine had no significant effect on immune capacity of patients.
Gautret et al. (March 20, 2020) published results of a limited trial which has been the genesis of much academic debate and criticism apropos of the use of HCQ to treat COVID-19. The researchers enrolled twenty-six COVID-19 positive patients who were PCR (polymerase chain reaction) documented SARS-CoV-2 carriers in nasopharyngeal sample at admission; and, 16 patients who refused treatment acted as negative controls. Six study patients were lost to follow-up.
Study patients received 600mg daily of HCQ (200mg three times a day). Nasopharyngeal viral load was tested daily in the hospital. Azithromycin was added if their clinical presentation indicated a need. The dose was 500mg on day 1 followed by 250mg per day for the next four days to prevent bacterial super-infection under daily electrocardiogram control.
Treated patients showed a significant reduction of the viral load at day 6-post inclusion compared to controls, as shown in the following graphs. Azithromycin added to HCQ was significantly more efficient for virus elimination.
Graph 1. PCR-positive samples in patients treated with HCQ (reproduced from Gautret et al)
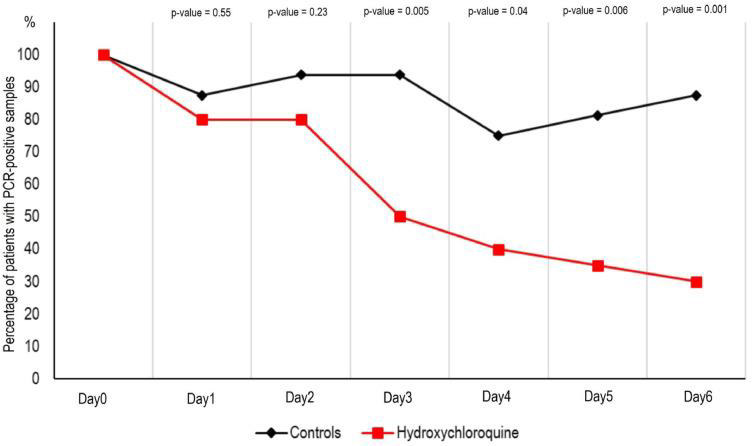
Percentage of patients with PCR-positive nasopharyngeal samples from inclusion to day 6 post-inclusion in COVID-19 patients treated with hydroxychloroquine and in COVID-19 control patients.
Graph 2. PCR-positive samples in patients treated with HCQ +/- azithromycin (reproduced from Gautret et al)
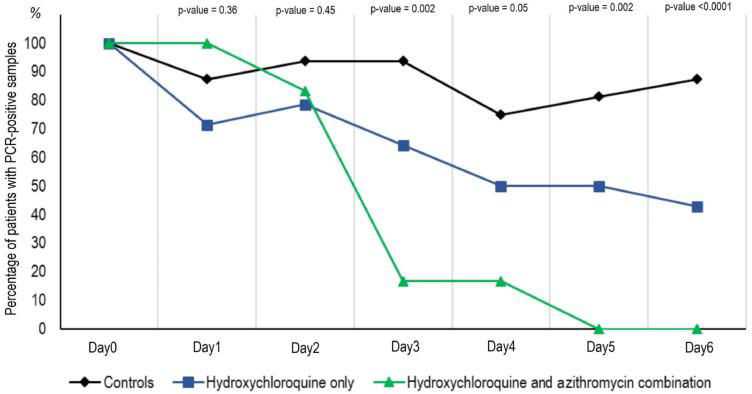
Percentage of patients with PCR-positive nasopharyngeal samples from inclusion to day 6 post-inclusion in COVID-19 patients treated with hydroxychloroquine only, in COVID-19 patients treated with hydroxychloroquine and azithomycin combination, and in COVID-19 control patients.
This research has been criticised by Frie and Gbinigie of the Oxford COVID-19 Evidence Service Team. Weaknesses identified include:
- A failure to recruit the 48 patients needed to achieve 85% power.
- Therapy effect sizes could therefore, be exaggerated and false-positives generated. One patient tested virus negative on day 6 but positive on day 8.
- There was insufficient medium to long-term follow-up.
- The trial was not randomised; hence, allocation bias could have been introduced.
Azithromycin and alternatives:
Azithromycin, a bacteriostatic macrolide, is active against gram-positive and Gram-negative bacteria including Bordetella pertussis and Legionella species plus Mycoplasma pneumoniae, and Haemophilus influenzae. Since the 1990s, resistance has been increasing to Streptococcus pneumoniae and Staphylococcus aureus.
The literature contains numerous reports that highlighted a statistically significant increase in arrhythmia risks and mortality.
Against these reports was a retrospective observational study of scripts dispensed between 2000 and 2011 in South Carolina. The volumes dispensed were: 283,743 azithromycin; 143,191 amoxicillin; 52,714 clindamycin; 38,133 clarithromycin and 49,734 for the quinolones. The researchers concluded, “Our study shows that the odds of cardiovascular mortality between azithromycin and other antibiotics are not statistically significantly different and previous published findings may not be applicable to the general population.”
Notwithstanding this variance of findings, the Australian Medicines Handbook carries a caution linked to a prolonged QT interval. This accords with a 2012 advisory from the US Food and Drug Administration (FDA) to consider the risk of fatal heart rhythms in those:
- With a prolonged QT interval (including congenital long QT syndrome);
- Taking medicines that are likely to prolong the QT interval; or
- With a history of torsades de pointes, arrhythmias or uncompensated heart failure.
Because of these cardiovascular concerns, other clinicians reported to use doxycycline as an alternative to azithromycin, to avoid these problems.
Heneghan et al (Oxford COVID-19 EMB team) offer the following treatment options for community-acquired pneumonia (CAP):
Early use of antibiotics in older adults
Non-response to initial antimicrobial therapy increases mortality, and so the initial selection of antimicrobials is critical. According to NICE [National Institute for Health and Care Excellence], to cover atypical and multiple pathogens in older patients with pneumonia and at risk of severe complications, the recommended choices of antibiotics in the community are:
| Amoxicillin with | 500 mg 3 times a day (higher doses can be used …[consult appropriate references]) for 5 days |
| Clarithromycin (if atypical pathogens) | 500 mg twice a day for 5 days |
Alternative oral antibiotics for penicillin allergy, if the pneumonia is of moderate-intensity; treatment should be guided by microbiological results when available.
| Doxycycline or | 200 mg on the first day, then 100 mg once a day for a further 4 days (5-day course in total) |
| Clarithromycin | 500 mg twice a day for 5 days |
As they note, “viral infections increase pneumococcal adherence to the local epithelium, facilitating bacterial infection. Adhesion of Streptococcus pneumoniae to epithelial cells, for example, is significantly enhanced by human coronavirus HCoV-NL63 infection.”
A more comprehensive treatment regime from Up To Date is supplied as a separate document for reference and guidance.
Autopsy findings:
The following may be of clinical significance to the treating respiratory physician.
Pathologic findings from these two patients were edema and prominent proteinaceous exudates, vascular congestion, and inflammatory clusters with fibrinoid material and multinucleated giant cells. Reactive alveolar epithelial hyperplasia was seen in case 1, and fibroblastic proliferation (fibroblast plugs) in case 2 is indicative of early organization. No prominent neutrophil infiltration was seen. The significance of the large protein globules is not entirely clear, as these were described in patients with SARS but could also represent a nonspecific change with aging. More cases with sufficient controls are necessary to further clarify this change.
The two cases reported here represent “accidental” sampling of COVID-19, in which surgeries were performed for tumors in the lungs at a time when the superimposed infections were not recognized. These provided the first opportunities for studying the pathology of COVID-19.
Conclusion:
COVID-19 has been a pandemic since March 11, 2020. Four patients from Seattle, Washington received a trial vaccine in March 17, 2020 and Australian researchers hope to begin human trials in late April 2020, but the best estimates for full production place vaccine availability at mid-2021.
Very limited data suggests a possible role for hydroxychloroquine, with the addition of azithromycin or, if there is a poor cardiovascular profile, the use of doxycycline. At best, the role for HCQ is equivocal, with a leaning towards a possible benefit.
Against that background, clinicians have an onerous challenge.
HCQ is a known drug, having received FDA approval for use in lupus and rheumatoid arthritis in 1956. However, COVID-19 is reported to cause liver and renal injury, and HCQ is metabolised in the liver and present in the urine. This may make reaching the therapeutic dose of HCQ a challenge and increases the risk of adverse drug reactions.
Frequent monitoring of liver function and renal function in patients with COVID-19 would be imperative, to minimise any further damage whilst seeking to attain a therapeutic drug level.
Until more trial results are available HCQ – with the appropriate antibiotics and supportive medical care – may be one of the few therapeutic options available to avert patient death.
It is a hard choice and must be underpinned by the medical dictum primum non nocere – First, do no harm.
However, another factor must be weighed in the scales: “You go to war with the army you have, not the army you might want or wish to have at a later time.”
References:
- Centers for Disease Control and Prevention. Coronavirus: common human coronaviruses. Washington: U.S. Department of Health and Human Services; 2020.
- Centers for Disease Control and Prevention. Coronavirus: human coronavirus types. Washington: U.S. Department of Health and Human Services; 2020.
- Centers for Disease Control and Prevention. Medicines for the Prevention of Malaria While Traveling Hydroxychloroquine (Plaquenil™). Washington: U.S. Department of Health and Human Services; 2020.
- Chou HW, Wang JL, Chang CH, Lai CL, Lai MS, Chan KA. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015; 60(4): 566-77.
- Colson P Rolain JM, Lagiera JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020; 105932.
- Cubero GI, Reguero JJ, JM Ortega. Restrictive cardiomyopathy caused by chloroquine. British Heart Journal 1993; 69: 451-2.
- Department of Health. What you need to know about coronavirus (COVID-19). Canberra: Australian Government; 2020.
- Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020; 105938.
- European Centre for Disease Prevention and Control. Q & A on COVID-19. ECDC: Frösunda; 2020.
- File TM. Treatment of community-acquired pneumonia in adults who require hospitalization. UpToDate; 2020.
- Finbloom DS, Silver K, Newsome DA, Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985; 12(4): 692-4.
- Frie K, Gbinigie K. Chloroquine and hydroxychloroquine: current evidence for their effectiveness in treating COVID-19. Oxford: University of Oxford; 2020.
- Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020; 14(1): 72-3.
- Gautret P, Lagier JP, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020; 105949.
- Grenfell R, Drew T. Here’s why it’s taking so long to develop a vaccine for the new coronavirus. Science Alert; 2020.
- Heneghan C, Aronson J, Hobbs R, Mahtani K. Rapidly managing pneumonia in older people during a pandemic. Oxford: University of Oxford; 2020.
- Huang M, Tang T, Pang P, Li M, Ma R, Lu J, et al. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020: doi. 10.1093.
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020; 55(3): 105924.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395(10224): 565–74.
- McMullen BJ, Mostaghim M. Prescribing azithromycin. Aust Prescr. 2015; 38: 87-9.
- R & D Systems. ACE-2: the receptor for SARS-CoV-2. Minneapolis: R & D Systems; 2020.
- Ratliff N, Estes ML, Myles JL, Shirey EK, McMahon JT. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N Engl J Med. 1987; 316:191-3.
- Rao GA, Mann JR, Shoaibi A, Bennett CL, Nahhas G, Sutton SS, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. 2014; 12(2): 121-7.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012; 366(20): 1881.
- Rossi S (ed). Australian Medicines Handbook. Adelaide: AMH; 2020.
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virology Journal 2019; 16: 69.
- Schroeder RL, Gerber JP. Chloroquine and hydroxychloroquine binding to melanin: some possible consequences for pathologies. Toxicology Reports 2014; 1: 963-8.
- Stokkermans TJ, Trichonas G. Chloroquine and hydroxychloroquine toxicity. Bethesda: National Center for Biotechnology Information; 2019.
- Sutton SS, Hyche S, Magagnoli J, Hardin JW. Appraisal of the cardiovascular risks of azithromycin: an observational analysis. J Comp Eff Res. 2017; 6(6): 509-17.
- Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020; doi:10.1016.
- Vandergriendt C. What is a coronavirus? San Francisco: Healthline; 2020.
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal. 2005; 2(69).
- World Health Organization. Rolling updates on coronavirus disease (COVID-19). Geneva: WHO; 2020.
- Yang P, Wang X. COVID-19: a new challenge for human beings. Cell Mol Immunol. 2020; doi.org/10.1038.
- Yazdany J, Kim A. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 2020; DOI: 10.7326/M20-1334.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270-3.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
