“Without urgent, coordinated action by many stakeholders, the world is headed for a post-antibiotic era, in which common infections and minor injuries which have been treatable for decades can once again kill.” These are the remarks made by Dr Keiji Fukuda, Assistant Director-General for Health Security, World Health Organization (WHO), as WHO presented the first global report on antibiotic resistance surveillance in 2014.
In 1940, the antibiotic era was launched after the discovery of penicillin and its subsequent mass production. Sir Alexander Fleming warned that the inappropriate use of antibiotics could lead to resistance. It did not take long for healthcare professionals to realise that antimicrobial resistance (AMR) was actually happening, as revealed by the WHO 2014 report on global surveillance of antimicrobial resistance.
We are indeed heading towards the era when treatable minor injuries or infections become life-threatening. The report predicts that the annual number of deaths attributed to AMR globally will increase from 700,000 to 10 million in 2050, more than the predicted value of 8.2 million deaths due to cancer.
Antimicrobial resistance
While many have acquaintances with microbes as pathogens through infection episodes or as the one causing diseases, humans actually share a relationship with microbes that is essentially beneficial. Since birth, humans are colonised by microbiota, formed by an assembly of microorganism communities. This naturally occurring interaction has multiple important functions in humans, from getting nutrients from food, mucosal structure differentiation, to even promoting the immune system.
Resistance development is described as a natural phenomenon of bacteria evolution and mutation. It is a protective mechanism for microorganisms to withstand toxic substances and to survive in the environment. This is often described as the Darwinian selection process through which the microorganisms are competing for survival. For instance, beta-lactamases, which hydrolyse and inactivate beta-lactam antimicrobials (e.g. penicillins), were already present for millions of years. Environmental microorganisms, such as soil microbes, already inherit the genetic factors to be multi-drug resistant. Permafrost sediment has been found to contain DNA with genes encoding resistance to beta-lactam, tetracycline and glycopeptide antibiotics. Sampling from the soil revealed that the majority of the soil bacteria strains could resist seven to eight out of 21 antibiotics tested, including synthetically made quinolones. The availability of the genes pool with the potential to express the resistance determinant is referred to as environmental antibiotic resistome, suggesting that the reservoir for antibiotic resistance lies in nature. However, the current selection of AMR is more likely attributed to antimicrobial consumption by humans and animals as well as agricultural use. This leads to the acquisition of resistant elements in susceptible pathogens from benign resistant microorganisms over time.
Antibiotic resistance: Mechanism and transmission
In general, antibiotics work by causing cell death or growth inhibition, and the subsequent reduction in pathogen population allows humans’ natural immune system to reign during the infection episode(s). The targets of the antibiotics are the bacterial cell wall, cell membranes, protein synthesis, nucleic acid synthesis and folic acid metabolism. On the other hand, there are also four general mechanisms by which resistant bacteria adapt: alteration of drug target or binding site, efflux systems, immunity and bypass, and enzyme catalysed destruction. Among these resistance mechanisms, energy-dependent efflux system is a common mechanism among microorganisms against most classes of antibiotics. For example, the ability to pump antibiotics out enables Staphylococcus aureus to resist fluoroquinolones and macrolide antibiotics such as erythromycin. Staphylococci also produce beta-lactamases, cleaving the beta-lactam ring of penicillin and cephalosporins. Target alteration by chromosomal mutation has altered the aminoglycoside binding site on the 30s subunits, reducing fluoroquinolones binding to DNA gyrase. The target of vancomycin is modified with a change in the cell wall structure.
Development and spread of resistance to antibiotics
Humans are colonised with microbiota. Upon exposure to antimicrobials, microorganisms with resistant genes survive and proliferate within the host, becoming the dominating colony. Subsequently, other colonising bacteria also acquire resistant genes from the mutated colony, leading to increased resistant populations in individual hosts. Moreover, increasing use of wide-spectrum cephalosporins and fluoroquinolones in patients reduces the intestinal microflora, increasing the colonisation by Clostridium difficile, which can cause severe infective diarrhoea or pseudomembranous colitis.

Figure 1. How antibiotic resistance happens (Image from Centers for Disease Control and Prevention)
Apart from mutation, the resistance genes can be transferred from one to another, not only within the same but also between different species. Of several mechanisms, the transmission of plasmids is one important way by which bacteria acquire resistance. Plasmids containing resistant genes, namely R genes, can pass from one bacterial cell to another via conjugation or connecting tubes between bacterial cells. Plasmids can also be packaged into bacteria-specific viruses and transferred via transduction, like in the case of staphylococci and streptococci strains. One of the classic examples of plasmid-mediated acquisition resistance is how Staphylococcus aureus became resistant to methicillin (an antibiotic designed to be the solution to penicillin resistance) within three years of use, resulting in methicillin-resistant Staphylococcus aureus (MRSA). Furthermore, MRSA is currently not just confined within hospitals, but also has spread to the community. Now the increasing use of antibiotics over the past decades has led to further growth of resistant bacterium to crisis levels such as extended spectrum beta-lactamases that inactivate not only penicillin but most cephalosporins and the rise of New Delhi metallo-beta lactamase (NDM-1) carbapenemases which overcome all beta-lactam antibiotics.
Apart from the transfer of resistance across strains of microorganisms, resistance can also be disseminated among humans and animals. Resistant enterobacteriaceae can be spread via faecal-oral route, especially in areas of poor sanitary practice. Contaminated healthcare workers’ hands can transfer MRSA from one patient to other patients. Sharing of personal items or activities with skin-to-skin contact also increase the spread of MRSA in the community. Veterinarians, livestock handlers and pet owners are also potential carriers of resistant organisms like MRSA.
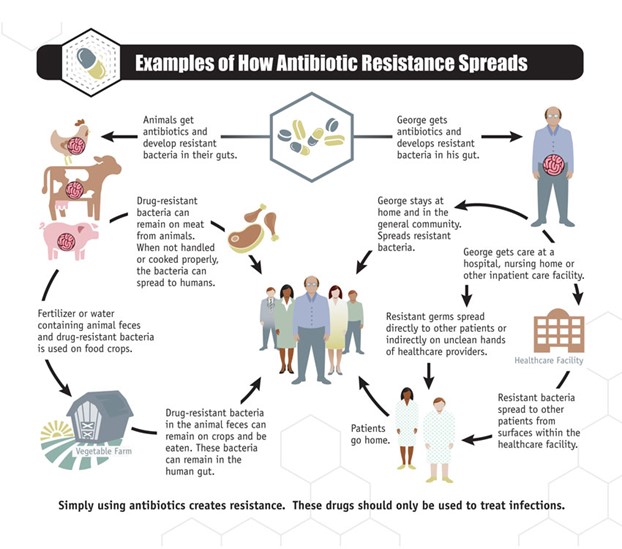
Figure 2. How antibiotic resistance spreads (Image from Centers for Disease Control and Prevention)
Resistance spreads geographically too. The ease of international travel nowadays has escalated the rate of human intestinal colonisation of resistant Enterobacteriaceae. Travelling to Asian countries, especially Southeast Asia, is identified to be an independent risk factor that subject tourists to be carriers of extended spectrum beta-lactamase (ESBL) Enterobacteriaceae. Furthermore, NDM-1 is no longer confined to India but has spread to other parts of the world like European countries.
The driver of AMR
Although resistance is part of the natural process for evolution and survival of microorganisms, the rate of resistant generations and the distribution are accelerated via the selection pressure exerted by the use of antimicrobials in human and its application in agriculture, veterinary and others. High rates of antibiotic use in hospitals increase the prevalence of nosocomial infections due to resistant organisms. Moreover, the intensive care setting may have a higher prevalence of infection by multidrug resistant organisms than non-intensive care settings.
A large part of antibiotic use actually comes from outpatient care. A study by US Centers for Disease Control and Prevention (CDC) researchers found that at least 30% of outpatients’ antibiotic prescriptions in the US are unnecessary and nearly half are prescribed for common viral infections such as colds, sore throats, bronchitis and sinus and ear infections. On the other hand, should antibiotics be warranted, the inappropriate selection of antibiotics and unnecessary broad-spectrum coverage is a concern too. Fifty percent of children in the US are prescribed broad-spectrum antibiotics such as azithromycin, amoxicillin/clavulanic acid and cephalosporins, instead of first-line narrow-spectrum antibiotics such as amoxicillin and penicillin, to treat common infections such as streptococcal pharyngitis and acute sinusitis. A meta-analysis of studies shows that antibiotic prescribing in primary care is associated with increased resistance rates. Moreover, after treatment for respiratory or urinary infection, patients can develop and even carry resistant bacteria until 12 months later. This review supports the call to avoid unnecessary prescribing or treat with the least number of antibiotic courses within the shortest period. On the other hand, as travelling to Asian countries is identified to be an independent risk factor that subjects tourists to be carriers of ESBL Enterobacteriaceae, self-treatment with antimicrobials increases the risk further to as high as 80%.
In addition, subjecting patients to prolonged duration of antibiotics at doses below the inhibitory concentration can induce AMR development. When a group of school children was given beta-lactam antibiotics for prolonged duration and/or doses lower than recommended, their pharyngeal carriage of penicillin-resistant Streptococcus pneumoniae increased. Moreover, the antibiotic dosing pattern and duration of treatment may not only affect efficacy but also resistance development. For example, smaller doses of ciprofloxacin at a higher frequency can select resistant Pseudomonas aeruginosa compared to the same total amount given as a single dose as shown by in vitro studies. This association of pharmacodynamic/pharmacokinetic properties of antibacterials and its impact on clinical outcomes, including resistance development, need to be explored further in the interest of prolonging the lifespan of currently available antibiotics.
Apart from use in the treatment of infectious diseases, it is worth noting that antimicrobials are also used as prophylaxis in humans and animals. However, the function as growth promoter, pest control in agriculture and plants, and other commercial uses such as household use has constituted a large part of antimicrobial utilisation. It is also important to note that disposal of antimicrobials or the sewage system may not be handled properly in certain parts of the world, hence polluting the environment and subsequently inducing resistant microorganisms.
Preserving the antimicrobials: Antimicrobial stewardship
Antibiotic resistance rates are escalating, yet the pipeline of new antibiotics is running dry. There have been efforts to identify new chemical molecules but most are not suitable to be developed into drugs. In addition, strict government regulatory requirements complicate and slow new drug registration/approval. Consequently, these challenges have hindered pharmaceutical companies from prioritising investment in antibiotic development. Consequently, the numbers of newly approved antibacterials have been declining. Linezolid and daptomycin are two new drugs introduced for gram-positive pathogens such as MRSA, but development of resistance has already been observed. In contrast, there are so few antibiotics available for use against gram-negative organism, forcing the need to resort to much older antibiotics like colistin which was not favoured earlier due to the side effects such as nephrotoxicity. Therefore, it becomes crucial for us to take steps to conserve the currently available antibiotics and optimise their appropriate use.
Although antimicrobial resistance is inevitable as part of a natural phenomenon, it is driven further by the volume of antimicrobial use. Recognising the trend of AMR, the 68th World Health Assembly in 2015 endorsed a global action plan with the aim to continue the work of preventing infectious diseases and to ensure that quality medicine is safe, effective, accessible and used optimally. The One Health approach, which engages all sectors, is essential to bring these forward and conserve antimicrobials through a stewardship programme.
The goal of antimicrobial stewardship (AMS) is to optimise clinical outcomes while minimising unintended consequences of antimicrobial use, including toxicity, the selection of pathogenic organisms and the emergence of resistance. The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) has defined AMS as “coordinated interventions designed to improve and measure the appropriate use of antimicrobial agents by promoting the selection of the optimal antimicrobial drug regimen including dosing, duration of therapy and route of administration.” The scope of the guidelines mainly focuses on the inpatient healthcare setting and the implementation requires team work with a clinical pharmacist as the core member.
In general, there are two core strategies of AMS. The first strategy is formulary restriction and preauthorisation which doctors need to seek approval before antibiotics can be prescribed. The second strategy is prospective audit and feedback (PAF) so that the prescriber is given suggestions or comments after antibiotics are initiated which can impact on de-escalation and duration of antibiotic therapy. A stewardship programme led by a pharmacist resulted in a reduction of antibiotic usage by as much as 64%, by performing PAF intervention three days a week in a community hospital. The IDSA guidelines also recommend that the programme can be further supplemented by strategies such as guidelines and clinical pathway, education, de-escalation or streamlining of antibiotic choice, antimicrobial order form, dose optimisation and parenteral to oral antibiotic switch. A systematic review of 37 studies conducted by Wagner and colleagues concluded that AMS programmes in hospitals improve prescribing and microbial outcomes without negative impacts on patient outcomes.
Antimicrobial stewardship in the outpatient setting
Although AMS has been historically focused on the inpatient setting, widespread implementation is necessary by extending the efforts to the outpatient setting, long-term care facilities and even the community. Since 2003, CDC has been promoting the appropriate use of antibiotics in the community through a programme named Get Smart: Know When Antibiotics Work. Several proven effective interventions have been recommended. In combination with education, audit of prescribing compliance to evidence-based practice and feedback to prescribers can help to improve antibiotic prescribing in outpatient settings. Clinical decision supports such as electronic support systems or clinical pathways for common infections also help to facilitate accurate diagnosis and promote appropriate antibiotic use. Delayed prescribing is an effective approach by which patients are not prescribed antibiotics immediately after their clinic visit for infections such as acute upper respiratory infection, but are advised to wait for one to two days or more. A randomised controlled trial shows that delayed prescribing resulted in less than 40% of patients receiving antibiotics and there was insignificant differences among symptom severity and duration and patient satisfaction among those receiving immediate and those receiving no or delayed antibiotic prescription. In addition, this has helped to manage patient perception and expectation on antibiotics for self-limiting infections. Other strategies include academic detailing and poster-based interventions. The display of poster-size letters at clinics stating the clinician’s commitment to not prescribing antibiotics inappropriately has been shown to reduce antibiotic use. A systematic review found that antimicrobial stewardship programmes in outpatient settings with interventions such as education, guideline implementation, delayed prescribing, communication skills training, decision support and laboratory testing are associated with a significant decreased use of antibiotics.
Role of pharmacists in antimicrobial stewardship
AMS requires teamwork. The inclusion of pharmacists as an integral member of the AMS team is an evidence-based recommendation for effective implementation of an AMS programme. With their professional expertise, pharmacists are well placed to optimise the antimicrobial agents use in patient care. Hospital pharmacists assist in antimicrobial selection, dose optimisation and adjustment according to patients’ organ function. Moreover, pharmacists can also advise on de-escalation to antimicrobials with a narrower spectrum and suggest switching from parenteral to suitable oral antibiotics with good bioavailability such as metronidazole. This is achieved by joining ward rounds, performing prescription review and providing feedback to prescribers. In addition, the participation of pharmacists in therapeutic committees helps to maintain formulary and drug use restriction or policy so that AMR development can be minimised. Pharmacists also perform surveillance and drug utilisation reports for clinical and economic analysis. Quantitative reports of antimicrobial usage in hospitals are reported as defined daily doses per 1,000 occupied bed-days and can be submitted for institutional or national antimicrobial utilisation surveillance programmes.
Preventing unintended effects of antimicrobials
It is also important to have pharmacists’ involvement in the effort of safe medication practice to reduce errors and adverse drug events associated with antimicrobials. Apart from the concern of resistance, antimicrobials can also cause complications of therapy unrelated to its antimicrobial activity, inducing immunological reactions or toxic effects. It is often the major group that cause adverse drug reactions. Hypersensitivity reactions triggered by the penicillin group are not uncommon and often present as fever, rash (maculopapular and urticaria) or anaphylaxis. Macrolides like azithromycin may cause nausea, vomiting, abdominal pain and diarrhoea. In addition, there are also adverse effects specific to the compound such as aplastic anaemia by chloramphenicol. Moreover, antimicrobials account for 19% of emergency visits for drug-related adverse events in US. Apart from genetic factors that make patients more vulnerable, the incidence of antibiotic side effects can be attributed to patients’ age, medical backgrounds and organ functions which often can be avoided by dose adjustment in consideration of drug pharmacokinetics. Pharmacists should always probe if patients have any history of allergies and also help to differentiate the nature of the allergic reaction so that first-line antibiotics can still be used instead of broad-spectrum second-line antibiotics.
Health education and self-care
As most antibiotics are prescribed in primary care, a greater outreach of national and international campaigns promoting antibiotic resistance awareness and rational use will require primary care providers’ involvement. Therefore, it is no doubt that community pharmacists should be a part of the move in promoting public awareness of antibiotic resistance. There is still a large gap in general public knowledge towards antibiotic resistance. A systematic review shows that half of the population do not know that antibiotics are not effective against viral infection, one third is not aware that misuse of antibiotics can promote resistance and almost half practice self-discontinuation of antibiotics when they feel better. Hence, pharmacists, often at direct point of contact, are in the key position to manage patients’ perceptions and expectations of antibiotics. As upper respiratory infections such as acute sinusitis, pharyngitis and bronchitis are often caused by viruses and do not need antibiotics, pharmacists should be able to identify and educate patients on the natural course of such infections and advise on effective self-care management. For example, for a common cold presenting with cough, runny nose and fever, pharmacists can suggest symptomatic relief with paracetamol and a decongestant. Equipping patients with proper self-management strategies and realistic expected duration of recovery helps to reassure them so that unnecessary clinic visits and antibiotic demands are reduced. Nevertheless, it is equally important for pharmacists to recognise when referral to doctors is warranted, such as populations that are immunocompromised and patients with organ failure. If antibiotics are necessary, counselling on the proper use of antibiotics should be provided. The “FRAIS” mnemonic can be used during counselling on antibiotic therapy:
Finish the course;
Regular intervals such as 6-hourly;
After, with or without food;
Interaction;
Side effects.
Patients should also be advised on the proper handling, return and disposal of excess antibiotics and to not share with others.
Public health promotion: Hand hygiene and vaccination
The vicious cycle of resistance development can be prevented by reducing or avoiding the use of antimicrobials. As proper hand hygiene and sanitation is crucial in preventing infection transmission and the spread of resistant organisms, pharmacists should encourage frequent hand washing during their encounters with patients. Furthermore, vaccination should be prioritised and emphasised as it can help to prevent infection and, hence decrease the need for antimicrobials. Increased uptake of influenza vaccination has shown to reduce the use of antibiotics by 10 prescriptions per 1,000 population in Canada. Pharmacists should be involved in screening and promoting routine immunisation among healthcare workers and educating the public on the significant value of vaccines. Therefore, it is essential for pharmacists to keep up to date with the national schedule of immunisation for children and special populations (e.g. the elderly and patients with asthma or chronic obstructive pulmonary disease or immunocompromised patients). Furthermore, public health promotion like smoking cessation to keep the immune system healthy is also a preventive care activity that pharmacists should engage in.
Conclusion
AMR is inevitable and the development is accelerated by the therapeutic and non-therapeutic application in various sectors. The 2014 WHO report has clearly stated that AMR is in an alarming state, leading to increasing clinical and financial burden to healthcare systems worldwide. This is a global concern that requires the One Health approach to tackle antimicrobial utilisation in various sectors including agriculture, veterinary and environment. Pharmacists are one of the key healthcare professionals in the efforts of tackling AMR. As the majority of the antibiotic use in medicine is from primary care, pharmacists in the community setting are well placed to be at the frontier of antimicrobial stewardship, in collaboration with prescribers and also patients. Combating AMR requires teamwork and every stakeholder should play a proactive role to ensure the appropriate use of antimicrobials. Otherwise, the post antibiotic era, when common infections become untreatable and life threatening, will soon become a reality.
Since publication of the 2014 report into AMR and surveillance, the WHO has established the Global Antimicrobial Resistance and Use Surveillance System (GLASS). This has expanded to include 109 countries and territories worldwide. The most recent report summarises the 2019 data that was provided to the WHO in 2020 and provides an overview of surveillance programs and recent developments in AMR.
References:
- ASHP statement on the pharmacist’s role in antimicrobial stewardship and infection prevention and control. Am J Health Syst Pharm. 2010; 67(7): 575–7.
- Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Executive Summary: Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62(10): 1197–202.
- Centers for Disease Control and Prevention. About Antibiotic Resistance. Washington: CDC; 2020.
- Centers for Disease Control and Prevention. CDC: 1 in 3 antibiotic prescriptions unnecessary. Washington: CDC; 2016.
- Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work. Washington: CDC; 2020.
- Centers for Disease Control and Prevention. Get Smart: Know When Antibiotics Work. Delayed Prescribing Practices. Washington: CDC; 2020.
- Centers for Disease Control and Prevention. Healthcare Infection Control Practices Advisory Committee (HICPAC). Epidemiology of MDROs. Washington: CDC; 2006.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096.
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010; 74(3): 417–33.
- Drekonja DM, Filice GA, Greer N, Olson A, MacDonald R, Rutks I, et al. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol. 2015; 36(2): 142–52.
- Fleming N, Barber S, Shiru-Oredope D. Pharmacists have a critical role in the conservation of effective antibiotics. Pharmaceutical J. 2011; 287: 465.
- Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013; 309(22): 2345–52.
- Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000; 21(1): 53–60.
- Gualano MR, Gili R, Scaioli G, Bert F, Siliquini R. General population’s knowledge and attitudes about antibiotics: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2015;24(1):2–10.
- Holloway KA, Ivanovska V, Wagner AK, Vialle-Valentin, D. Ross-Degnan. Have we improved use of medicines in developing and transitional countries and do we know how to? Two decades of evidence. Trop Med Int Health. 2013; 18(6): 656–64.
- Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387(10014): 176–87.
- Kantele A, Lääveri T, Mero S, Vilkman K, Pakkanen SH, Ollgren J, et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis. 2015; 60(6): 837–46.
- Lim V. Antibiotic Stewardship. IeJSME. 2012; 6(Suppl 1): S75–S79.
- Mandell GL, Bennett JE, Dolin R. . Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. United States: Churchill Livingstone Elsevier; 2010.
- Olofsson SK, Cars O. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin Infect Dis. 2007; 45(Suppl 2): S129–36.
- Rang HP, Dale MM. Rang & Dale’s Pharmacology. Edinburgh: Churchill Livingstone; 2011.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008; 47(6): 735–43.
- Wagner B, Filice GA, Drekonja D, Greer N, MacDonald R, Rutks I, et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol. 2014; 35(10): 1209–28.
- World Health Organization. AMR: Global Report on Surveillance 2014. Geneva: WHO; 2014.
- World Health Organization. Antimicrobial resistance. Geneva: WHO; 2020.
- World Health Organization. The role of pharmacist in encouraging prudent use of antibiotics and averting antimicrobial resistance: a review of policy and experience. Copenhagen: WHO Regional Office for Europe; 2014.
- Wright GD. Q&A: Antibiotic resistance: where does it come from and what can we do about it? BMC Biol. 2010; 8:123.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
