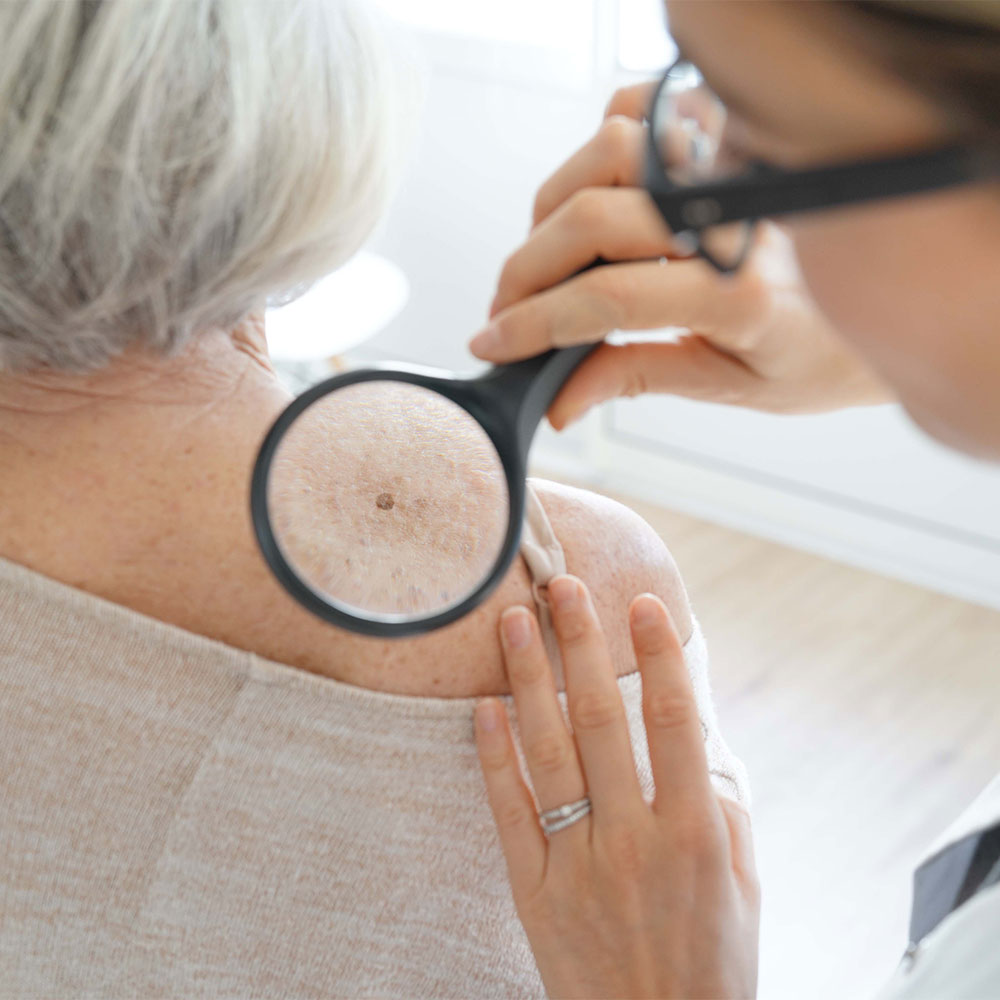
Evidence suggests that hydrochlorothiazide is associated with an increased risk of carcinoma in sun-exposed tissues. It is theorised that this may be due to the photosensitising properties of hydrochlorothiazide.
A recent Australian study demonstrated that hydrochlorothiazide use was associated with a 2.6-fold greater risk of lip cancer and a 20% increased risk of cutaneous melanoma. The risk also appeared to be dose-related, as patients with high hydrochlorothiazide use (defined as a cumulative dose of ≥ 25,000mg) demonstrated a 4.7-fold higher odds of lip cancer. This echoes the findings of two Danish case-control studies that showed an increased risk of squamous cell carcinoma and basal cell carcinoma with hydrochlorothiazide use. This increased risk was not observed for furosemide, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin II antagonists.
Hydrochlorothiazide is a thiazide diuretic indicated for the treatment of hypertension and oedema. It is available as a single ingredient tablet and is also formulated with other antihypertensive agents in a range of fixed-dose combination products. Patients taking hydrochlorothiazide should be advised to regularly check their skin and lips and promptly report any new or changed skin lesions or moles to their doctor. Patients should also limit their sun exposure and practice sun safety.
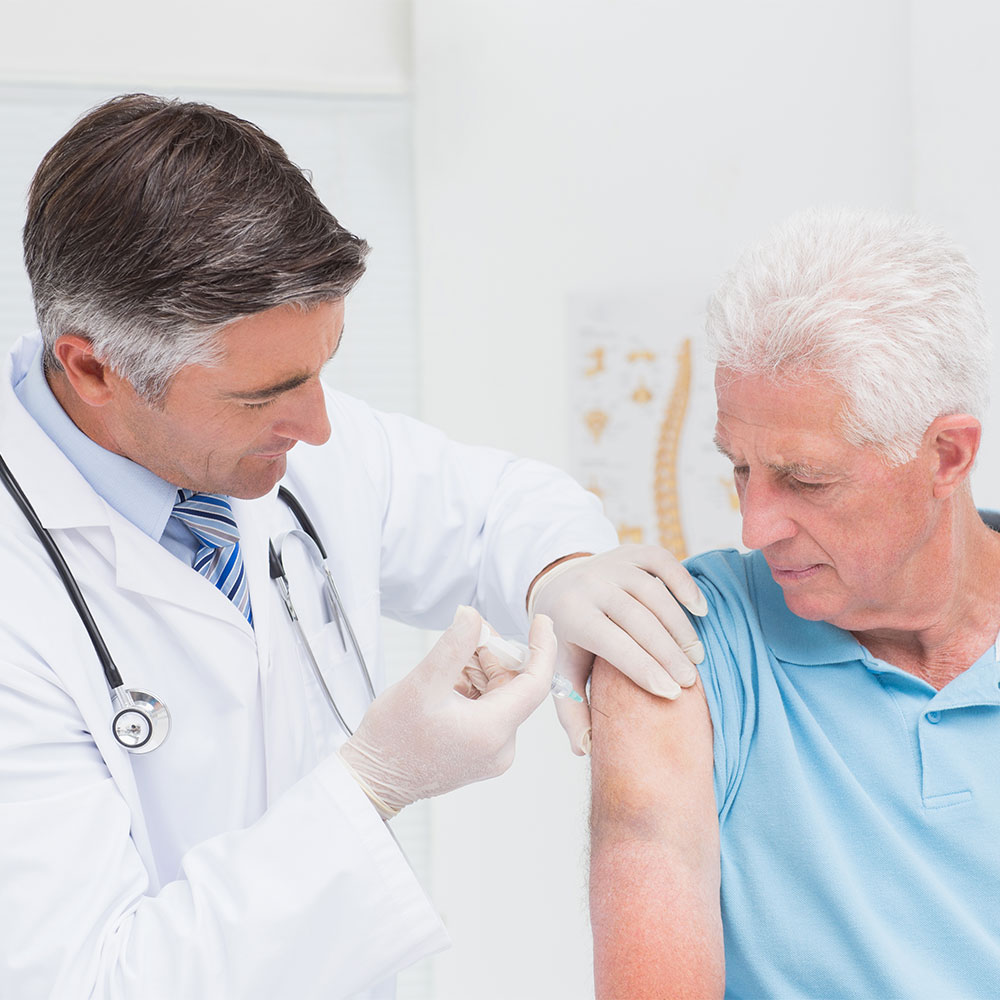
The Therapeutic Goods Administration (TGA) has issued a safety advisory regarding the use of Zostavax® in people with compromised immune function. Zostavax® is a live, attenuated varicella-zoster vaccine indicated for the prevention of herpes zoster in people aged 50 years and older. This vaccine is contraindicated in people with primary and acquired immunodeficiency states as well as those receiving immunosuppressive therapy. However, the product information emphasises that it is not contraindicated in people receiving topical or inhaled corticosteroids, low-dose systemic corticosteroids, or corticosteroid replacement therapy.
The TGA warning follows a new report of death due to disseminated varicella-zoster virus (Oka vaccine strain) infection. This patient was taking hydroxychloroquine and low-dose prednisolone when they received the zoster vaccine, in line with clinical recommendations. The TGA reminds healthcare professionals that this potentially life-threatening condition can occur in patients on low-dose immunosuppressive therapy and highlights the importance of a risk-based assessment prior to Zostavax® administration.
The Australian Immunisation Handbook can be referred to for further information regarding zoster vaccine use in people taking immunosuppressive therapy and the value of serological testing.


Semaglutide is the latest glucagon-like peptide-1 (GLP-1) analogue to be added to the Pharmaceutical Benefits Scheme (PBS). GLP-1 is an incretin hormone that stimulates the release of insulin and inhibits the release of glucagon in a glucose-dependent manner. Semaglutide is indicated for the treatment of type 2 diabetes mellitus.
In a 30-week double-blind clinical trial, the mean baseline glycated haemoglobin (HbA1c) level fell from 68mmol/mol (8.4%) to 48mmol/mol (6.5%) in the group receiving semaglutide 1mg weekly, compared to 67mmol/mol (8.3%) in the placebo group. A target HbA1c of <53mmol/mol (7.0%) was achieved in 79% of the semaglutide 1mg group compared to 11% of the placebo group.
Semaglutide is administered weekly as a subcutaneous injection. Gastrointestinal adverse effects such as nausea, vomiting, and diarrhoea are commonly experienced. These effects are usually mild to moderate in severity and reduce as therapy continues. Weight loss is commonly observed due to reduced appetite. Body weight reductions of ≥5% may be achieved in around two-thirds of patients, while losses of ≥10% occur in a quarter of patients. A reduction in the dose of concomitant insulin or sulfonylurea therapy may be required when starting semaglutide to reduce the risk of hypoglycaemia. Pancreatitis has been associated with the use of GLP-1 analogues. While the risk is thought to be low, patients should be counselled to seek immediate medical attention if they experience unexplained severe abdominal pain.
Evolocumab is now available on the Pharmaceutical Benefits Scheme (PBS) for high-risk atherosclerotic cardiovascular disease (ASCVD) in non-familial hypercholesterolaemia.
The PBS criteria must include low-density lipoprotein (LDL) levels >2.6mmol/L, symptomatic ASCVD with additional risk factors and the patient must have had ≥ 12 weeks of optimised therapy.
In the FOURIER trial, evolocumab was demonstrated to significantly reduce LDL cholesterol levels by 59% compared to placebo.
Evolocumab significantly reduced the risk of the primary composite endpoint (hazard ratio 0.85) of cardiovascular death, myocardial infarction, hospitalisation for unstable angina or coronary revascularisation, as well as key secondary composite endpoint (hazard 0.80) of cardiovascular death, myocardial infarction or stroke.
Adverse effects include injection side reactions, nausea, back or joint pain, nasopharyngitis, the common cold, flu-like symptoms, and in rarer situations, hypersensitivity reactions including angioedema.


Trifluridine/tipiracil is now available on the Pharmaceutical Benefits Scheme (PBS) for pre-treated cancer of the stomach or gastro-oesophageal junction.
In the TAGS trial, trifluridine/tipiracil was demonstrated to significantly prolong overall survival in pre-treated gastric cancer (5.7 months vs 3.6 months) compared to placebo, with 21% of treated patients alive at 1 year.
The disease control rate was 44% for patients who had a complete or partial response to trifluridine/tipiracil, where time of deterioration of ECOG (Eastern Cooperative Oncology Group) performance status almost doubled (4.3 months vs 2.3 months) compared to placebo.
The incidence of treatment-related adverse effects was consistent with that seen for metastatic colorectal cancer, with haematological adverse effects markedly higher, including neutropenia, anaemia, leucopenia and thrombocytopenia.


The Pharmaceutical Benefits Scheme (PBS) has recently expanded the listing criteria for Symbicort®. Symbicort® contains the corticosteroid, budesonide plus the long-acting β2-agonist (LABA), formoterol.
The new PBS listing allows Symbicort® to be used as reliever therapy in the management of mild asthma in patients 12 years of age and older who are not currently using a single agent LABA. Previously, Symbicort® was only PBS listed for the treatment of moderate to severe forms of asthma, as well as chronic obstructive pulmonary disease (COPD).
This new PBS listing aligns with the recommendations of the Global Initiative for Asthma which no longer recommends the use of a short-acting β2-agonist alone for adults and adolescents with asthma. Instead, an inhaled corticosteroid plus LABA is their preferred option for symptom relief in these patients. In patients with mild asthma, randomised controlled trials demonstrate that this therapy is associated with a 64% lower rate of severe exacerbations compared to treatment with a short-acting β2-agonist alone.
The following table shows the PBS streamlined authority codes for the different presentations of Symbicort®. Please refer to the PBS website for full clinical criteria.
| Product |
PBS Authority Streamlined Codes |
| Presentation |
Strength |
Mild asthma |
Asthma |
COPD |
| Symbicort® Rapihaler® |
50mcg/3mcg |
|
4397 |
|
| 100mcg/3mcg |
10482 |
4397 |
|
| 200mcg/6mcg |
|
4404 |
10121 |
| Symbicort® Turbuhaler® |
100mcg/6mcg |
|
4380 |
|
| 200mcg/6mcg |
10464 |
7970 |
|
| 400mcg/12mcg |
|
7979 |
10121 |
From 1st July 2020, Lantus® products will no longer be available on the Pharmaceutical Benefits Scheme (PBS). Lantus® contains insulin glargine, a long-acting insulin analogue that is indicated for the management of type 1 and type 2 diabetes mellitus. It is presented as pre-filled pens (Lantus® SoloStar®) and cartridges (Lantus®).
Insulin glargine 100 units/mL will remain on the PBS in the form of Optisulin® and Semglee®. Optisulin® is the same formulation as Lantus® and is available in the same presentation and devices, i.e. the SoloStar® pre-filled pen device, and cartridges for use in the AllStar Pro® or JuniorStar® reusable pens. Semglee® is a biosimilar of Lantus® and is only available in a pre-filled disposable pen device.
Dose adjustments are not required when switching from Lantus® to Optisulin®. Patients should continue to administer their dose at the same time of day and monitor their blood glucose levels as directed by their healthcare professional.
An overview of insulin glargine products available on the PBS from 1st July 2020 is shown in the table below.
| |
Optisulin® |
Semglee® |
Toujeo® |
| Strength |
100units/mL |
100units/mL |
300units/mL |
| Form |
3mL cartridges
3mL pre-filled pen |
3mL pre-filled pen |
1.5mL pre-filled pen |
| Formulation compared to Lantus® |
Identical formulation |
Biosimilar |
N/A |
| Equivalence with Lantus® for the purposes of PBS substitution |
Equivalent
(pre-filled pens ‘a’ flagged, cartridges ‘b’ flagged) |
Equivalent
(pre-filled pens ‘a’ flagged) |
N/A |
The Pharmaceutical Benefits Scheme (PBS) has recently made changes to the listings of some commonly prescribed antibiotics. These updates are in response to a Department of Health review in which one in five repeat antibiotic prescriptions were found to be dispensed more than 30 days after the original prescription. The Pharmaceutical Benefits Advisory Committee (PBAC) recommended changes to reduce unnecessary repeat prescriptions and improve antimicrobial stewardship.
The antibiotics affected are amoxicillin, amoxicillin with clavulanic acid, cefalexin, and roxithromycin. From 1st April 2020, these listings were amended so that the maximum quantity corresponded with a full treatment course for specific indications. New listings for greater quantities and repeats were created that required a valid streamlined authority code. In many of these cases, prolonged oral antibiotic therapy was only subsidised for patients initiated on intravenous therapy.
From 1st May 2020, these new streamlined authority listings were further updated so that prolonged antibiotic therapy is now available to patients regardless of whether treatment was initiated using oral or intravenous therapy.
The PBS website should be consulted for complete clinical criteria.


Neuraminidase inhibitors are a class of medications used in the management of influenza A and B infection. Neuraminidase is an enzyme found on the surface of the influenza virus that is important for the movement of virus particles into target cells and the subsequent release of new virus particles from infected cells. Blocking this enzyme, therefore, inhibits viral replication within the body.
There are currently three neuraminidase inhibitors registered in Australia: oseltamivir, zanamivir, and peramivir. Peramivir is a newer agent that is administered as a single intravenous infusion. It is indicated for the treatment of acute influenza in adults and children from two years of age within 48 hours of symptom onset.
A large double-blind, double-dummy randomised controlled study compared the safety and efficacy of peramivir and oseltamivir. Subjects with confirmed influenza A or B were randomly assigned to receive a single infusion of peramivir (300mg or 600mg) or a five-day course of oseltamivir. This study concluded that peramivir is non-inferior to oseltamivir for the primary endpoint of time to alleviation of symptoms. In regards to safety, the overall incidence of adverse reactions was lower in the peramivir groups, although the rate of severe adverse reactions was similar across all groups. Surveillance data may be consulted for current information on local epidemiology and resistance profile of the circulating influenza strain.
A brief overview of the different neuraminidase inhibitors is shown in Table 1.
| |
Peramivir |
Oseltamivir |
Zanamivir |
| Indications |
Treatment of influenza |
Treatment and prevention of influenza A and B |
| Approved population |
Adults and children ≥ 2 years |
Adults and full-term neonates |
Adults and children ≥ 5 years |
| Route |
IV infusion |
Oral |
Inhalation |
| Typical adult treatment dose |
600mg |
75mg twice daily |
2 inhalations twice daily |
| Duration of treatment |
Single-dose |
5 days |
| Presentation |
Vial
|
Capsules
Oral liquid |
Rotadisks + Diskhaler device |


The coronavirus disease (COVID-19) pandemic is presenting a range of challenges to healthcare professionals. One of the biggest issues in any rapidly evolving space is the ability to access current and reliable information.
A number of peak Australian health professional groups have collaborated to form the National COVID-19 Clinical Excellence Taskforce. This new taskforce aims to translate emerging research and data into practical, evidence-based guidelines. The first set of national clinical guidelines for the care of people with COVID-19 is now available. These guidelines are referred to as “living guidelines” as they will be continuously revised and updated as new evidence comes to light. Clinicians are encouraged to submit key questions online to notify the taskforce of areas that require further guidance.
This may also be an opportune time for healthcare professionals to refresh their knowledge and understanding of infection control principles. The Australian Commission on Safety and Quality in Healthcare provides a suite of eLearning modules supported by the Infection Prevention and Control Workbook. These modules cover topics such as hand hygiene and the use of personal protective equipment. All content is based upon the Australian Guidelines for the Prevention and Control of Infection in Healthcare (2019). Consistent implementation of these guidelines will improve patient outcomes and ensure a safer environment for all healthcare workers.




















