HPS Pharmacies wish to advise of a potential safety issue regarding Oxycodone Sandoz® 5mg modified release tablets as follows:
Oxycodone Sandoz® Modified Release Tablets
Oxycodone hydrochloride 5mg
ARTG 153605
The individual foil blister strips no longer contain the words ‘modified-release tablets’. This may present a medication safety risk as it is not obvious that the tablets are modified-release when looking at the foil strips as shown below. The words ‘modified-release’ are also less prominent on the outer carton as highlighted below.
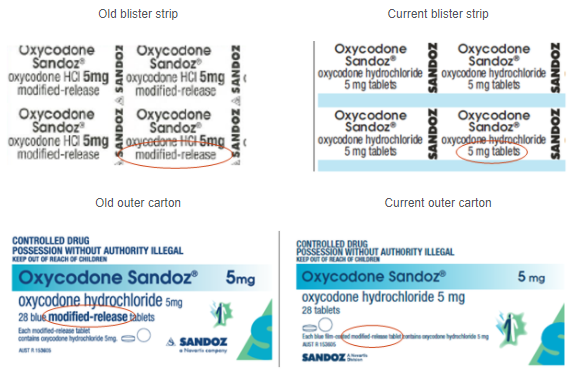
Oxycodone Sandoz® 5mg modified release tablets are blue and are not scored, while immediate-release oxycodone tablets are white and have a score line. Modified-release tablets must be swallowed whole and should not be broken, chewed, or crushed. To avoid confusion, it is recommended that all blister strips are stored in their original carton.
HPS Pharmacies advise that no quality, safety or efficacy issues have been identified with the tablets themselves.
Due to the potential risk of confusion, Sandoz has commenced the process of again updating the labelling of all modified-release oxycodone medicines. New designs, more prominently featuring the words ‘modified-release’ are expected to be introduced within the next six months.
Retain this notice in a prominent position, including in other related business units for one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Sandoz on 1800 726 369 or your pharmacist at HPS Pharmacies.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
