HPS Pharmacies wish to advise that Vertex, in consultation with the Therapeutic Goods Administration (TGA), has issued a product defect correction notice for Trikafta® as follows:
Trikafta® Tablets
Elexacaftor / Tezacaftor / Ivacaftor
ARTG 330423
This notice has been issued due to the potential for some packets to contain the correct tablets in the incorrect orientation. In some cases, the orange morning doses are located in the evening dosing section of the wallet, and the blue evening dose is found in the morning section, as shown below.
Figure 1. Incorrect orientation of tablets
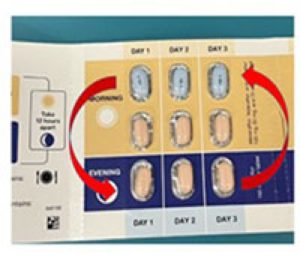
If patients were to take the medication as presented in the affected packets, they would receive the same daily dose but in a different dosing schedule. It is important that patients receive this medication as prescribed in order to prevent recurrence of their underlying disease.
The only affected batch number is 1846070 (expiry 03/2023).
The TGA advises healthcare professionals to check all packs from the affected batch to ensure that tablets are correctly orientated. Any product affected by this issue should be quarantined until returned to the supplier.
Retain this notice in a prominent position, including in other related business units for one month. Report any problems identified with medicines, vaccines or medical devices to the TGA.
Should you require further information regarding this matter, please contact Vertex on 1800 179 987 or your pharmacist at HPS Pharmacies.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
