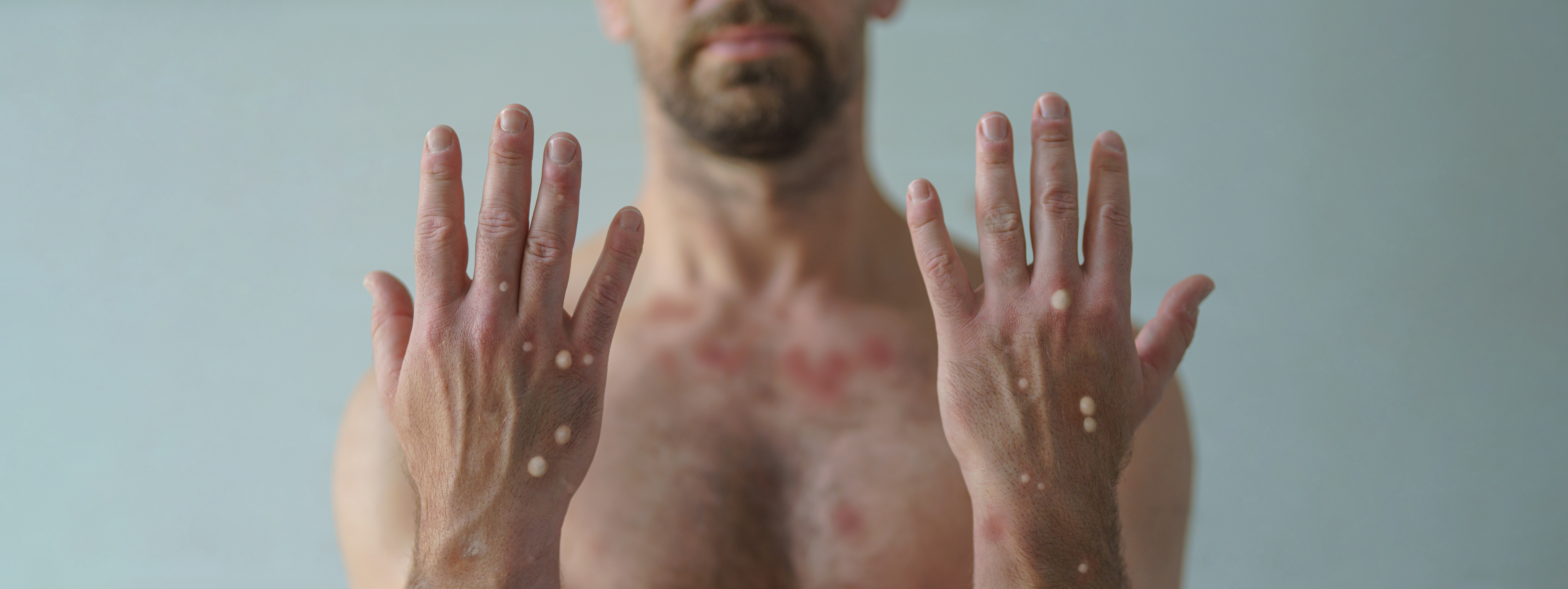
Monkeypox has been declared a Communicable Disease Incident of National Significance in Australia. As of 16 August 2022, 82 probable or confirmed cases of monkeypox have been reported to the National Notifiable Diseases Surveillance System (NNDSS).
Monkeypox is a zoonotic viral disease endemic in several African countries. In countries where monkeypox is endemic, outbreaks have historically been restricted to rural populations living within or adjacent to tropical rainforests. However, since May 2022, the World Health Organization (WHO) has received reports of monkeypox from 12 member states that are not endemic for the virus and in people with no established travel links to endemic areas.
Monkeypox infection
The virus that causes monkeypox belongs to the orthopoxvirus genus of the Poxviridae family. This is the same genus as the smallpox virus.
The incubation period for monkeypox is thought to be around 7-14 days but may be as long as 21 days. Patients may experience a prodromal phase with fever, headache, and fatigue before the characteristic rash appears. The lesions are often deep and evolve from macular to papular, vesicular, and pustular before crusting. The lesions may be painful in the initial stages and then itchy once they have crusted over. Pitted scars or pigmentation changes may remain after the lesions have healed.
The severity of infection varies significantly. Some people may develop a single lesion, while others develop multiple lesions which may coalesce and result in large regions of skin disease. Complications such as secondary infections, bronchopneumonia, sepsis, encephalitis, and infection of the cornea with vision loss can also occur. People at higher risk of severe infection include people who are immunocompromised, pregnant women, infants and young children.
While smallpox and monkeypox share some similarities, monkeypox has a much lower fatality rate. The WHO reports that the recent case fatality ratio for monkeypox is around 3-6%. However, the current global outbreak is seeing few deaths. This may be due to the reporting of mild cases that were previously not diagnosed, different demographics affected, or a less deadly strain circulating.
Isolation
The infectious period is thought to extend from the onset of rash until all scabs have healed. Human-to-human transmission may occur following close physical contact during this infectious period. Transmission is via contact with lesions, body fluids (including respiratory droplets), and contaminated materials (such as towels and bedding).
People with a suspected infection should isolate until a negative result is obtained; people with confirmed infection should isolate until the infectious period is over. Household members should avoid physical contact with the infected person and objects that have been in contact with the infected person (e.g. towels). Careful hand and respiratory hygiene are important. If the infected person cannot isolate alone, a face mask should be worn when they are around other people.
Vaccination
As smallpox and monkeypox are closely related, smallpox vaccines can protect against monkeypox. Studies suggest that vaccination against smallpox provides around 85% effectiveness against monkeypox.
There are two smallpox vaccines available in Australia:
- Jynneos® is a live vaccine produced from an attenuated, non-replicating orthopoxvirus. The vaccine is administered subcutaneously as a two-dose schedule given at least 28 days apart. This vaccine is considered a third-generation vaccine. As it is non-replicating, it is safe for people with compromised immune systems or atopic dermatitis. It is associated with fewer adverse effects compared to earlier smallpox vaccines.
- Jynneos® is not currently registered with the Therapeutic Goods Administration (TGA). However, it is available under section 18A of the Therapeutic Goods Act 1989 (exemption because of emergency). Limited supplies have been secured by the Commonwealth and some States and Territories.
- Acam2000™ is also a live-attenuated vaccine. It is administered via the percutaneous route using 15 jabs of a bifurcated needle. A single dose is required, although revaccination may be considered every three years for people at continued high risk of exposure to live pox viruses. Acam2000™ is a second-generation vaccine that is replication competent. It must not be used in pregnancy, severe immunocompromise, or active atopic dermatitis. Patients must also be advised of the risk of self-inoculation and spread to close contacts due to viral shedding. The virus sheds from the cutaneous lesion that forms at the site of inoculation from around day three until scabbing occurs. During this time, accidental infection of skin at other sites (self-inoculation) or infection of close contacts can occur. To avoid this, the vaccination site must be kept covered during this period of viral shedding.
- Acam2000™ is registered with the TGA and included in the National Medical Stockpile.
The Australian Technical Advisory Group on Immunisation (ATAGI) provides guidance on the use of these vaccines. The current advice is that Jynneos® is preferred for both pre-exposure prophylaxis and post-exposure prophylaxis. This is due to the more favourable safety profile and simpler method of administration.
The global supply of Jynneos® is limited, and demand is high. The overall risk of contracting monkeypox in Australia is currently low and widespread vaccination is not recommended. Initial access to the vaccine will prioritise groups at higher risk. The key risk groups identified by ATAGI include:
- Anyone categorised by public health authorities as a high-risk monkeypox contact in the past 14 days;
- Gay, bisexual and other men who have sex with men (GBMSM) with a high number of sexual contacts;
- Sex workers (especially those whose clients are in high-risk categories);
- Anyone in the above risk categories who is planning to travel to a country experiencing a significant outbreak (vaccination recommended 4-6 weeks before travel); and
- Immunisation providers who administer the ACAM2000™ vaccine.
Treatment
Monkeypox is generally a self-limiting condition. Most people will not require treatment other than simple supportive care such as over-the-counter analgesics. In some cases, complications of monkeypox may require treatment, e.g. antibiotics for the management of secondary cellulitis.
Specific therapy for monkeypox may be considered in the following cases:
- People presenting with severe disease (e.g., haemorrhagic disease, confluent lesions, sepsis, encephalitis, or other conditions requiring hospitalisation); and
- People at risk of severe disease.
- Immunocompromised
- Children (especially those <8 years of age)
- Pregnant or breastfeeding
- People with one or more complications
Two treatment options are tecovirimat and vaccinia immunoglobulin. These agents are investigational, and their use requires a thorough assessment of the risks and benefits.
Tecovirimat
Tecovirimat is an orally-active antiviral indicated for the treatment of monkeypox, cowpox, and smallpox. It can also be used for the treatment of complications following vaccination against smallpox. The efficacy of tecovirimat is based on animal studies. In non-human primates, tecovirimat demonstrated a significant mortality benefit (95% survival in the tecovirimat group, compared to 5% in the placebo group). A Phase 1 study conducted in healthy human volunteers did not identify any safety concerns. Adverse events commonly reported in the study included headache, nausea, and diarrhoea.
Tecovirimat is available in 200mg capsules. The usual adult dose is 600mg twice daily for 14 days. Weight-based dosing advice is available for children ≥13kg in the guidelines. Dose adjustment is not required for renal or hepatic failure.
While tecovirimat is not approved by the TGA, it is currently the preferred first-line drug option for severe monkeypox infection according to the Australian Human Monkeypox Treatment Guidelines. Supplies of tecovirimat are held in the National Medical Stockpile.
Vaccinia immunoglobulin
Vaccinia immunoglobulin is the second-line choice for the treatment of monkeypox, although it may be preferred in pregnant patients.
Vaccinia immunoglobulin is also the first-line option for the treatment of complications following vaccination with a replication-competent vaccinia vaccine (i.e. Acam2000™). Indications for the use of this product following vaccination include:
- Eczema vaccinatum (widespread pustular or erosive lesions from the vaccinia virus, occurring particularly in areas affected by atopic dermatitis);
- Generalised vaccinia (results from viraemia and presents with a generalised vaccinia virus rash which may be accompanied by fever, myalgia, and headache. This reaction is self-limiting in immunocompetent hosts);
- Progressive vaccinia (presents with delayed or absent local wound healing and widespread lesions originating from the inoculation site that can become necrotic. The risk is highest in severe immunocompromise);
- Vaccinia infections in individuals who have skin conditions; and
- Atypical infections induced by vaccinia virus (excluding isolated keratitis).
Vaccinia immunoglobulin is not considered to be effective in the treatment of postvaccinial encephalitis.
Vaccinia immunoglobulin is administered as an intravenous infusion. The usual dose is 6,000 U/kg, given as soon as symptoms appear. Repeat doses may be required depending on symptom severity and response to treatment. Higher doses may be considered if the initial response is poor.
Common adverse reactions reported in clinical trials include headache, nausea, rigors, and dizziness.
Caution is required when monitoring blood glucose in patients receiving vaccinia immunoglobulin. This product contains maltose which some blood glucose monitoring systems falsely interpret as glucose. Clinical decisions based on incorrect blood glucose readings can lead to serious events. Therefore, only glucose monitors and test strips that are glucose-specific should be relied upon during treatment with vaccinia immunoglobulin.
Other potential therapies
Cidofovir is a potential therapeutic agent. While there is currently no human data to support its use in the treatment of monkeypox, in vitro and animal studies show efficacy against orthopoxviruses. Cidofovir is associated with significant adverse effects, which may outweigh the potential benefits.
Cidofovir is currently TGA registered for the treatment of cytomegalovirus (CMV) retinitis in patients with acquired immunodeficiency syndrome (AIDS). The Australian Human Monkeypox Treatment Guidelines advise careful consideration of the use of cidofovir for monkeypox in order to preserve supply for patients with difficult-to-treat CMV disease and other viral infections in transplant patients.
Brincidofovir is another potential therapy for monkeypox. This antiviral is not registered by the TGA. It is approved by the US Food and Drug Administration (FDA) for the treatment of smallpox. There is currently no human data to support its use in the treatment of monkeypox. Brincidofovir has shown efficacy against orthopoxviruses in in vitro and animal studies. It is currently undergoing clinical trials in Australia.
Summary
Most people are not at high risk of contracting monkeypox, and mass vaccination is not recommended. Vaccine supplies are currently limited and will be directed toward people at the highest risk of contracting the infection.
For most people, monkeypox is a self-limiting infection that does not require specific treatment. However, severe disease can occur, and treatment may be deemed necessary. The Australian Human Monkeypox Treatment Guidelines should be referred to for information on specific therapies.
The monkeypox situation is evolving in Australia and health advice may change. The Department of Health can be accessed for current medical advice, official reports, and case numbers.
References:
- Acam2000™ smallpox vaccine (live vaccinia virus) Australian approved product information. Sydney: Emergent Sales and Marketing. Approved June 2020.
- Australian Government. Australian Human Monkeypox Treatment Guidelines. Canberra: Department of Health; 2022.
- Australian Technical Advisory Group on Immunisation. Updated ATAGI clinical guidance on vaccination against monkeypox. Version 2.0. ATAGI; 2022.
- Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 13(10): e0007791.
- CNJ-016, Vaccinia Immune Globulin Intravenous (Human). United States approved prescribing information. Cangene. Approved January 2010.
- Grosenbach DW, Honeychurch K, Rose EA, Chinsangaram J, Frimm A, Maiti B, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018; 379(1): 44–53.
- Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin N Am. 2019; 33: 1027-43.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
