Background
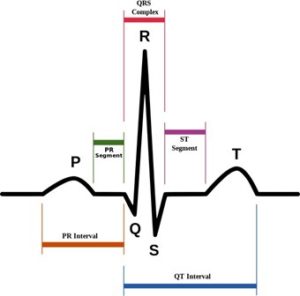
An electrocardiogram (ECG or EKG) is a non-invasive test that is used to evaluate electrical activity of the heart. The P wave represents atrial depolarisation which occurs prior to atrial contraction. The QRS complex represents ventricular depolarisation which occurs prior to ventricular contraction. The T wave represents ventricular repolarisation. The duration of time between the onset of ventricular depolarisation and end of repolarisation is described as the QT interval.
Figure 1. Normal sinus rhythm as seen on ECG (Credit: Agateller (Anthony Atkielski) licensed under Public Domain via Wikimedia Commons)
A prolonged QT interval on the ECG is used as a marker for increased risk of Torsades de Pointes, a potentially life-threatening form of polymorphic ventricular tachycardia. In some cases, brief episodes may self-terminate. However, in severe cases, it may cause sudden cardiac death. Torsades de Pointes is associated with a high mortality risk of 10%.
Hospital patients are at greater risk of drug-induced QT interval prolongation and Torsades de Pointes, due to the increased likelihood of being prescribed high-risk medications during an admission. Additional risk factors include concomitant heart disease, advanced age, electrolyte imbalances, bradycardia and kidney or liver disease.
Importantly, drug-induced QT interval prolongation can be preventable. Understanding the risk factors, medications related to QT interval prolongation and assessing the risk is essential to preventing poor clinical outcomes.
QT drugs
Certain medications are associated with varying risk levels of Torsades de Pointes. Medications considered as conditional risk are only associated with Torsades de Pointes under certain conditions of use (e.g. overdose, hypokalaemia, drug interactions).
The risk of developing QT interval prolongation can also be affected by the administration method, such as in cases of rapid IV dosing.
Table 1. Examples of medications and associated risk of Torsades de Pointes (source: Credible Meds)
| Class | Known Risk | Possible Risk | Conditional Risk |
| Antibiotics | Moxifloxacin
Clarithromycin Roxithromycin Azithromycin Ciprofloxacin Erythromycin |
Norfloxacin | Metronidazole |
| Antidepressants | Escitalopram
Citalopram |
Nortriptyline
Mirtazapine Venlafaxine |
Amitriptyline
Doxepin |
| Antifungal | Fluconazole | Voriconazole
Ketoconazole |
|
| Antipsychotic | Haloperidol
Droperidol Chlorpromazine |
Clozapine
Asenapine Lurasidone |
Olanzapine
Risperidone Ziprasidone |
| Antiemetic | Droperidol
Chlorpromazine Ondansetron |
Granisetron
Promethazine |
Metoclopramide |
| Anaesthetic | Cocaine
Propofol |
||
| Antiarrhythmic | Sotalol
Flecainide Amiodarone |
||
| Analgesic | Methadone | Tramadol
Buprenorphine |
|
| Other | Hydroxychloroquine | Levetiracetam |
Assessing QT interval prolongation risk
The Tisdale Risk Score for QT interval prolongation may be a useful predictive tool to assess the risk in hospitalised patients.
The use of the Tisdale Risk Score was studied in a cardiac care unit, in which 13% of alerts resulted in additional ECG/laboratory monitoring or treatment of modifiable risk factors and discontinuation of 17.9% of medication orders.
Figure 2. Tisdale Risk Score for QT interval prolongation
| Risk Factor | Points |
| Age ≥ 68 years | 1 |
| Female sex | 1 |
| Loop diuretic | 1 |
| Potassium Serum K+≤ 3.5 mEq/L | 2 |
| Admission QTC ≥450 ms | 2 |
| Acute myocardial infarction | 2 |
| 1 QTC interval-prolonging drug | 3 |
| ≥ 2 QTC interval-prolonging drugs | 3 |
| Sepsis | 3 |
| Heart failure | 3 |
| Maximum risk score | 21 |
Risk score category: Low risk = <7; Moderate risk = 7 to 10; High risk = >11
Role of Pharmacist
Pharmacists can help minimise the risk of QT interval prolongation and Torsades de Pointes in hospitalised patients through:
- Awareness of risk factors
- Monitoring serum electrolyte concentrations
- Identifying high-risk drug interactions
- Providing dose adjustments for renally cleared QT interval prolonging drugs in renally impaired patients
References:
- Risk Categories for Drugs that Prolong QT & induce Torsades de Pointes (TdP) [Internet]. Credible Meds; 2013 [cited 2022 Aug 25].
- Tisdale JE. Drug-induced QT interval prolongation and torsades de pointes: Role of the pharmacist in risk assessment, prevention and management. Can Pharm J (Ott). 2016; 149(3): 139-52.
- Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013; 6(4): 479-87.
- Tisdale JE, Jaynes HA, Kingery JR, Overholser BR, Mourad NA, Trujillo TN, et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014; 7(3): 381-90.
- Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019; 15: 105-14.
Subscribe Knowledge Centre Updates
Enter your details to receive Knowledge Centre updates
